Ketorol is an effective pain reliever. The medicine belongs to the group of anti-inflammatory non-steroidal drugs. In addition to the analgesic effect, the drug reduces inflammation and swelling. In addition, ketorol, the instructions indicate this, has antipyretic properties.
Compound
Composition of tablets: ketorolac (10 mg/tablet), MCC, lactose, corn starch, colloidal silicon dioxide, Mg stearate, Na carboxymethyl starch (type A).
The film shell contains: hypromellose, propylene glycol (additive E1520), titanium dioxide; dyes brilliant blue (22%) and quinoline yellow (78%) - olive green.
Composition of the solution: ketorolac (30 mg per milliliter), octoxynol, EDTA, Na chloride, ethanol, propylene glycol (additive E1520), Na hydroxide, water for injection.
Gel composition: ketorolac (20 mg per gram of gel), propylene glycol (additive E1520), dimethyl sulfoxide, carbomer 974P, Na methyl and propyl parahydroxybenzoate, tromethamine (trometamol), Drimon Inde flavoring, ethanol, glycerol, purified water.
Release form
- Tablets in p/o 10 mg, package No. 20. Ketorol tablets (INN - Ketorolac) are biconvex, round in shape, covered with a green coating (the core is white or close to white). On one side there is an imprint in the shape of the letter “S”.
- Solution for IM and IV administration 30 mg/ml, package No. 10. Ketorol injections are available in 1 ml ampoules, each of which has a breaking point and a ring in the upper part.
- Gel 2% for external use, tubes 30 g, packaging No. 1. Ketorol gel is a homogeneous, characteristically smelling, transparent (or translucent) substance.
Composition, forms and effects of the drug
The strong analgesic effect of the drug is provided by the main active substance - ketorolac tromethamine. Other components may vary depending on the manufacturer, so be sure to read the instructions.
The effect after taking the medicine is comparable to the effect of morphine. But at the same time, ketorol is a safer drug to use. The product can be used to combat different types of pain, the causes of which are any pathological conditions. It is used to eliminate symptoms and to treat specific diseases that are accompanied by swelling and fever.
The drug is produced in the form of:
- Tablets in a green soluble shell (with the amount of active substance - 10 mg).
- Transparent solution for intramuscular and intravenous administration (with an amount of active substance -30 mg/ml).
- Transparent, homogeneous gel (2%).
The most popular is the tablet form. After taking the drug, the active substance is completely absorbed into the gastrointestinal tract in the shortest possible time. Wherein:
- The maximum concentration in the blood is reached after an hour.
- Antipyretic and analgesic effects are observed after 30 minutes.
- Fatty foods slow down the absorption of the active substance.
Pharmacodynamics and pharmacokinetics
What is ketorolac?
The active substance Ketorol is a mixture in which the [+]R and [-]S enantiomers are present in equal quantities, and the analgesic effect is due to the [-]S form.
Pharmacodynamics
The drug is a powerful analgesic with anti-inflammatory properties and moderate antipyretic activity .
Its mechanism of action is associated with the ability to indiscriminately inhibit the activity of the COX 1 and 2 enzyme, mainly in peripheral tissues. As a result, the biosynthesis of Pg, a modulator of thermoregulation, pain sensitivity and inflammation, slows down.
Ketorolac does not affect opiate receptors, does not have anxiolytic or sedative effects, does not depress respiration, and does not lead to the development of drug dependence.
The analgesic effect of the drug is more pronounced in comparison with analogues. The effect of its use is comparable to the analgesic effect of morphine .
After injection into the muscle and oral administration, the pain begins to decrease after 0.5 and 1 hour, respectively. To achieve the maximum effect, it takes from 60 to 120 minutes.
Pharmacokinetics
Absorption in the digestive canal when taken orally is rapid. TSmax after taking 1 tablet on an empty stomach - 40 minutes.
Fatty foods reduce the Cmax in the blood while simultaneously increasing the TCmax up to 1 hour 40 minutes.
99% of the dose taken is bound to plasma proteins. With hypoalbuminemia, the concentration of free substance in the blood increases.
Bioavailability is one hundred percent (regardless of the method of administration of the drug).
When administered parenterally, the substance is absorbed quickly and completely.
Equilibrium concentration of Css when Ketorol is administered intravenously or intramuscularly at a dose of 120 mg/day. (4 injections of 30 mg) and when taken orally at a dose of 40 mg/day. (1 tablet in 4 doses) is achieved after 24 hours. The highest values of this indicator are observed with parenteral administration of 1 ml of solution 4 times a day.
The substance passes into breast milk: when a nursing woman takes 1 tablet of Tmax ketorolac in milk - 2 hours.
More than 50% of administered ketorolac is biotransformed in the liver with the formation of pharmacologically inactive metabolites . The substance is excreted mainly in the form of glucuronic metabolites and p-hydroxyketorolac. 91% of the dose taken is excreted by the kidneys, 6% - with the contents of the intestines.
In patients with healthy kidneys, the elimination half-life averages 5.3 hours.
In older people, T1/2 lengthens, in young people it shortens.
The functional state of the liver does not affect the pharmacokinetics of the drug. In case of kidney damage, in which the plasma concentration of creatinine is 19-50 mg/l, the half-life is extended to 10.3-10.8 hours; if renal failure is more severe, this figure exceeds 13.6 hours.
It is not excreted during hemodialysis .
Dosage and recommendations for treatment
In the postoperative period, tablets are usually prescribed, the price of which is affordable. This especially applies to cases where the instructions for use exclude ketorol injections against the background of certain consequences of operations. To relieve pain of any etiology, take one tablet. In case of intense severe pain, repeated use is allowed up to 4 times a day with a break of 4-6 hours. The duration of treatment is 5 days.
Ketorol tablets, the instructions for use emphasize this, should not be taken for any stomach problems. In this case, you need to purchase ketorol injection ampoules. In particular, intramuscular administration is indicated for persistent vomiting or difficulty swallowing. When they ask ketorol injections what they help with, experts often indicate radiculitis. In this case, the pain caused by this pathology can be dealt with quickly. Injections can be repeated with a break of 4 hours.
Ketorol gel is applied in a thin layer to clean and dried skin in areas of pain. Then rub in with soft massage movements. Procedures can be repeated 3-4 times a day until pain is relieved. The break between them is at least 4 hours. The duration of use of the gel is no more than 10 days.
Indications for use
Ketorol tablets: what does the tablet form of the drug help with?
The medicine helps reduce moderate/severe pain and inflammation, but does not affect the progression of the disease.
Tablets are effective for toothache , headaches , pain that occurs during menstruation, after injuries, in the postoperative and postpartum periods, against the background of cancer, with damage to peripheral nerves, with radiculopathy , arthralgia , myalgia , sprains, dislocations, rheumatic diseases .
What does injection medicine help with?
Ketorol in ampoules, like the tablet form of the drug, is used to relieve pain of moderate and severe intensity.
Parenteral administration of the drug is preferable in situations where it is necessary to quickly relieve pain, and also if the patient cannot take it orally (for example, with a peptic ulcer or due to the gag reflex).
Indications for use of Ketorol: what is the gel used for?
Local application of the gel helps reduce pain and inflammation in:
- injuries (inflammation and bruises of soft tissues, including after injury; bursitis , synovitis , ligament damage, epicondylitis , tendonitis );
- myalgia;
- arthralgia;
- radiculitis;
- neuralgia;
- rheumatic diseases.
Indications and contraindications for use
In order not to cause harm, it is important to know what ketorol helps with. Most often, ketorol, the instructions for use confirms this, is used as a symptomatic remedy to relieve headaches, dental and menstrual pain, as well as pain in the joints. In complex therapy, the drug is prescribed for the development of cancer and in the postoperative period.
The medicine is indicated to alleviate conditions in the following pathologies:
- Rheumatoid diseases.
- Spinal diseases and radiculitis.
- Ligament ruptures and muscle sprains.
Ketorol, like any non-steroidal drugs, is prohibited for use in the presence of diseases of the gastrointestinal tract. Otherwise, the risk of gastric bleeding increases. The drug is not prescribed to patients under 16 years of age or during pregnancy and lactation. You should stop using the medicine if you are hypersensitive to the components of the drug, as well as if you have the following pathologies:
- Bronchial asthma.
- Hyperkalemia.
- Heart failure.
- Liver and kidney diseases.
- Stroke.
There are other diseases for which the doctor recommends taking the drug with caution. These are chronic hypertension, diabetes mellitus, various nervous system disorders, etc.
Ketorol, for which a prescription is required, should not be taken with a number of other medications:
- Pentoxifylline.
- Acetylsalicylic acid.
- Anticoagulants.
- Lithium salts.
- Probenecid.
When taking the drug simultaneously with alcoholic beverages, the risks of intoxication of the body increase.
Contraindications
Contraindications for parenteral administration and oral administration of Ketorol:
- hypersensitivity to the components of the solution/tablets;
- complete or partial combination of clinical manifestations of aspirin-induced bronchial asthma (NSAID intolerance, asthma attacks, polypous rhinosinusitis );
- the presence of erosions and ulcerative defects on the mucous membrane of the upper gastrointestinal tract;
- bleeding in the active phase (gastrointestinal, cerebrovascular or other);
- worsened inflammatory bowel disease;
- hemophilia and other pathologies of the hemostatic system;
- end stage of heart failure (decompensated heart failure);
- functional disorders or active liver disease;
- confirmed hyperkalemia ;
- postoperative period after CABG;
- renal failure , in which the creatinine does not exceed 30 ml/min, progressive kidney pathologies ;
- pregnancy , childbirth, lactation ;
- age up to 16 years.
Relative contraindications:
- congestive heart failure ;
- bronchial asthma (BA);
- IHD;
- hypersensitivity to NSAIDs;
- pathological dys- or hyperlipidemia ;
- cerebrovascular pathologies;
- arterial hypertension;
- kidney damage, in which the creatinine is below 60 ml/min;
- sepsis;
- cholestasis;
- edema syndrome;
- diabetes;
- SLE;
- chronic obliterating diseases of the arteries of the lower extremities;
- treatment with other NSAIDs, anticoagulants , antiplatelet agents , SSRIs, oral corticosteroids;
- history of ulcerative lesions of the digestive canal;
- smoking;
- old age (over 65 years);
- alcohol abuse;
- severe somatic diseases.
External use of Ketorol is contraindicated in case of hypersensitivity to any of the components of the gel, aspirin asthma , eczema , weeping dermatitis , after the 27th week of pregnancy and during lactation . The gel is not intended for the treatment of open wounds and infected abrasions. The drug is prescribed to adolescents from the age of sixteen.
Ketorol gel should be used with caution in case of hepatic porphyria (at the stage of exacerbation of the disease), severe liver/renal failure , CHF, asthma, in pregnant women (in the 1st and 2nd trimesters) and in the elderly.
Side effects
More than 3% of patients with parenteral administration and oral administration of Ketorol experience:
- diarrhea and gastralgia (especially in older people with a history of peptic ulcer disease);
- dizziness , increased drowsiness , headache ;
- swelling (fingers, feet, ankles, legs, face);
- weight gain.
Somewhat less frequently (in 1-3% of patients) the following were recorded:
- flatulence , stomatitis , feeling of fullness in the stomach, vomiting, constipation ;
- increased blood pressure ;
- purpura, rash (including maculopapular) on the skin;
- pain and/or burning sensation at the injection site;
- increased sweating .
Rare side effects that occur in less than 1% of patients:
- nausea;
- formation of erosions on the gastrointestinal mucosa or its ulceration (including with perforation and/or bleeding, the symptoms of which are burning, pain and spasm in the epigastric region, bloody vomiting (coffee grounds), melena, heartburn , nausea, etc.);
- hepatomegaly;
- hepatitis;
- acute inflammation of the pancreas;
- surge arrester;
- cholestatic jaundice;
- lower back pain (sometimes accompanied by azotemia and/or hematuria );
- renal failure dominating the clinical picture , as well as hemolytic anemia and thrombocytopenia ;
- change in urine volume (decrease or increase);
- frequent urination;
- edema of renal origin;
- nephritis;
- hearing loss, tinnitus ;
- visual impairment (blurred perception of visual images);
- rhinitis , bronchospasm , laryngeal edema;
- aseptic meningitis;
- hyperactivity (restlessness, mood swings);
- depression;
- mental disorders;
- hallucinations;
- sudden loss of consciousness;
- edema ;
- swelling of the tongue;
- leukopenia , eosinophilia , anemia ;
- bleeding (from the lower gastrointestinal tract, nose, postoperative wound);
- exfoliative dermatitis;
- Lyell's syndrome , malignant exudative erythema , urticaria ;
- anaphylactoid reactions or anaphylaxis ;
- fever.
When ketorolac , peeling of the skin at the site of application of the gel, urticaria , and itching .
When applying the drug to a large area of the body, the possibility of developing systemic effects cannot be excluded, including:
- ulceration of the mucous membrane of the digestive canal;
- heartburn , diarrhea , vomiting, nausea, gastralgia ;
- increased activity of liver aspartate and alanine aminotransferase;
- dizziness , headache ;
- hematuria;
- fluid retention;
- hypersensitivity reactions;
- prolongation of bleeding time;
- anemia , leuko- and thrombocytopenia , agranulocytosis .
Ketorol®
Often - more than 3%, less often - 1-3%, rarely - less than 1%.
From the digestive system:
often (especially in elderly patients over 65 years of age with a history of erosive and ulcerative lesions of the gastrointestinal tract) - gastralgia, diarrhea; less often - stomatitis, flatulence, constipation, vomiting, feeling of fullness in the stomach; rarely - nausea, erosive and ulcerative lesions of the gastrointestinal tract (including with perforation and/or bleeding - abdominal pain, spasm or burning in the epigastric region, melena, vomiting like “coffee grounds*”, nausea, heartburn and etc.), cholestatic jaundice, hepatitis, hepatomegaly, acute pancreatitis.
From the urinary system:
rarely - acute renal failure, lower back pain with or without hematuria and/or azotemia, hemolytic uremic syndrome (hemolytic anemia, renal failure, thrombocytopenia, purpura), frequent urination, increased or decreased urine volume, nephritis, edema of renal origin.
From the senses:
rarely: hearing loss, ringing in the ears, visual impairment (including blurred vision).
From the respiratory system:
rarely: bronchospasm or dyspnea, rhinitis, laryngeal edema (shortness of breath, difficulty breathing).
From the CHC side:
often - headache, dizziness, drowsiness; rarely - aseptic meningitis (fever, severe headache, convulsions, stiffness of the neck and/or back muscles), hyperactivity (mood changes, anxiety), hallucinations, depression, psychosis.
From the cardiovascular system:
less often - increased blood pressure, rarely - pulmonary edema, fainting.
From the hematopoietic organs:
rarely - anemia, eosinophilia, leukopenia.
From the hemostasis system
: rarely - bleeding from a postoperative wound, nosebleeds, rectal bleeding.
From the skin:
less often - skin rash (including maculopapular rash), purpura, rarely - exfoliative dermatitis (fever with or without chills, redness, thickening or peeling of the skin, swelling and/or tenderness of the tonsils), urticaria, Stevens-Johnson syndrome, Lyell's syndrome.
Local reactions
Less common: burning or pain at the injection site.
Allergic reactions:
rarely - anaphylaxis or anaphylactoid reactions (change in facial skin color, skin rash, urticaria, skin itching, tachypnea or dyspnea, swelling of the eyelids, periorbital edema, shortness of breath, difficulty breathing, heaviness in the chest, wheezing).
Others
: often - swelling (face, legs, ankles, fingers, feet, weight gain); less often - increased sweating, rarely - swelling of the tongue, fever.
Instructions for use of Ketorol
Ketorol tablets: instructions for use
The medicine is taken orally from 1 to 4 times a day. A single dose is one tablet. The highest daily dose is four tablets. Treatment should last no more than 5 days in a row.
When switching from parenteral administration to the tablet form of the drug, the total dose of the drug in the form of tablets and solution on the day of transfer should not exceed 90 mg/day if the patient is under 65 years of age, and 60 mg/day if the patient is older than the specified age.
The upper limit of the daily dose of Ketorol in tablets on the day of transition is 30 mg.
Ketorol injections: instructions for use
The solution is intended for intramuscular and intravenous administration. The drug in this dosage form must be used in minimally effective doses.
If necessary, Ketorol can be used in combination with narcotic analgesics (the latter are prescribed in reduced doses).
For patients under 65 years of age, provided that their weight is more than 50 kg, no more than 2 ml of solution can be injected into the muscle once (including oral administration). As a rule, to relieve pain, 1 ml of Ketorol is administered in ampoules every six hours.
Ketorol is administered intravenously in 1 ml doses so that the volume of medication administered over five days does not exceed 15 single doses.
For patients weighing less than 50 kg, as well as patients with chronic renal failure, a single dose when administering Ketorol into the muscle should not exceed 1 ml of solution (including oral administration).
As a rule, the drug is administered in 0.5 ml doses so that the patient receives no more than 20 single doses in five days.
No more than 0.5 ml of solution can be administered intravenously to a patient with chronic renal failure or weighing less than 50 kg every six hours (within five days, no more than 20 single doses).
The upper limit of the daily dose for parenteral administration of Ketorol for patients under 65 years of age weighing more than 50 kg is 90 mg, for patients with chronic renal failure and patients weighing less than 50 kg is 60 mg.
The drug can be used for no more than 5 days in a row.
The IV solution should be administered over at least 15 seconds. Ketorol is injected intramuscularly deep into the muscle and also slowly.
The drug begins to act half an hour after administration. Maximum pain relief is observed an hour or two after the injection.
Ketorol Gel: instructions for use
The gel (ointment) should be applied to washed and dried skin. A single dose of the medicine is a column 1-2 cm long. Ketorol is distributed on the surface of the most painful area with soft massaging movements 3-4 times a day.
Re-use of the drug is possible no earlier than after 4 hours.
The gel can be used no more than 4 times a day. Do not exceed the recommended dose.
If after 10 days of treatment with Ketorol the patient’s condition does not improve or the pain and inflammation intensify, it is necessary to stop using the drug and seek medical help.
Without consulting a doctor, the gel can be used for no more than 10 days.
Additionally
If the drug is used in combination with narcotic analgesics (solution, tablets or suppositories), the dose of the latter may be reduced.
Instructions for use KETOROL
Ketorol is intended for intramuscular injection; the drug should not be used for epidural or spinal administration. The solution is injected slowly IM (deep into the muscle). The onset of the analgesic effect is about 30 minutes with its maximum severity within 1-2 hours, the average duration of analgesia is 4-6 hours.
Administration of the drug several times a day for more than 2 days is not recommended, since in most cases patients do not require longer-term analgesic therapy, or can be switched to oral ketorolac. In this case, the duration of use of ketorolac parenterally and orally should not exceed 5 days in total.
To achieve maximum analgesic effect in the early postoperative period, it is possible to use ketorolac and narcotic analgesics together; the daily dose of the latter in this case is reduced. Ketorolac does not affect the addiction of copioids and does not increase the associated respiratory depression or sedation.
Dose selection and adjustment should be made in accordance with the intensity of pain and response to the drug. To minimize side effects, it is recommended to use the minimum effective dose for the shortest possible course of treatment.
Adults:
The usually recommended initial dose of Ketorol is 10-30 mg, followed by 10-30 mg every 4-6 hours. In the early postoperative period, it is permissible to administer the drug every 2 hours, if necessary. The maximum daily dose is 90 mg/day, in patients with body weight less than 50 kg - no more than 60 mg/day.
Elderly patients (over 65):
It is recommended to use the drug in a dose of 10-15 mg every 4-6 hours, the total dose should not exceed 60 mg/day. Due to the higher risk of side effects in this group of patients, the minimum possible duration of treatment and regular monitoring of the patient’s condition to exclude gastrointestinal bleeding are recommended.
Children:
The safety and effectiveness of ketorolac in children have not been confirmed; the drug is not recommended for use in children under 16 years of age.
Patients with impaired renal function:
the use of ketorolac is contraindicated in patients with severe and moderate renal impairment. In case of mild renal dysfunction, it is permissible to use Ketorol in a dose of no more than 60 mg/day.
If it is necessary to combine parenteral and oral administration of Ketorol, the total daily dose should not exceed 90 mg (60 mg in persons over 65 years of age, with a body weight of less than 50 kg or impaired renal function), while the dose of the drug taken orally should not exceed 40 mg/day . It is recommended to quickly transfer the patient only to the oral form of the drug.
Overdose
Symptoms of overdose for parenteral use and oral administration: nausea, abdominal pain, vomiting, renal dysfunction, erosive lesions and ulceration of the gastrointestinal mucosa, metabolic acidosis .
If signs of intoxication , the victim is given gastric lavage and enterosorbents . Further treatment is symptomatic. ketorolac to be sufficiently removed from the body.
Cases of overdose with external use of the drug have not been described. If you accidentally ingest the gel, you should empty your stomach (induction of vomiting and taking an enterosorbent ) and consult a doctor.
Interaction
Drug interactions for Ketorol solution and tablets
The use of ketorolac in combination with other NSAIDs, ASA ( acetylsalicylic acid ), ethanol, calcium preparations, corticotropin and corticosteroids can lead to ulceration of the gastrointestinal mucosa and the development of bleeding from ulcerative defects.
Co-administration with Methotrexate increases hepato- and nephrotoxicity, and with Paracetamol - nephrotoxicity. With Methotrexate, ketorolac can be prescribed only if Methotrexate is used in the lowest possible dose (in this case, plasma concentrations of methotrexate ).
Probenecid reduces the volume of distribution and plasma clearance of ketorolac , increases its plasma concentration and increases the half-life. With the use of ketorolac, Methotrexate may decrease , and the toxicity of these substances may increase.
Use in combination with indirect anticoagulants and drugs for thrombolytic therapy , Heparin , cephalosporin antibiotics , antiplatelet agents increases the risk of bleeding.
Ketorolac , acting as an inhibitor of Pg synthesis in the kidneys, reduces the effect of diuretics and antihypertensive drugs .
In the case of simultaneous use with narcotic painkillers, the doses of the latter may be significantly lower.
Antacids do not alter the absorption of ketorolac .
Increases the hypoglycemic effect of oral antidiabetic agents and insulin , which requires dose recalculation.
Combined use with valproic acid provokes a violation of platelet aggregation.
Increases plasma concentrations of Nifedipine and Verapamil .
When combined with other nephrotoxic drugs (for example, with Au preparations), the likelihood of nephrotoxic effects increases.
Drugs that block tubular secretion reduce clearance and increase plasma concentrations of ketorolac .
Drug interactions with topical use of ketorolac
The possibility of pharmacokinetic interaction with drugs competing for binding to plasma proteins cannot be ruled out.
The gel should be used with caution in combination with Phenytoin , other NSAIDs, diuretics , Digoxin , Cyclosporine , Li drugs, Methotrexate , antidiabetic and antihypertensive drugs .
Patients using any of the listed drugs should begin treatment with Ketorol only with the approval of a physician.
special instructions
The choice in favor of one or another dosage form is made taking into account the indications for use and the intensity of pain.
Pills:
Use of Ketorol for more than five days in a row and/or at a dose exceeding the maximum allowable increases the risk of adverse reactions.
The drug should not be prescribed simultaneously with other NSAIDs, since simultaneous use with them leads to cardiac decompensation, fluid retention, and increased blood pressure .
The effects due to the effect of ketorolac on platelet disappear after 24–48 hours.
Ketorolac can change the properties of platelets, but the drug does not replace the preventive effect of ASA in pathologies of the heart and blood vessels.
To reduce the likelihood of developing NSAID gastropathy , the drug should be taken with Omeprazole , Misoprostol , and antacids .
Before prescribing the solution, you should find out whether the patient has previously had an allergic reaction to the drug or other NSAIDs. Due to the risk of hypersensitivity reactions, the first dose is administered under close medical supervision.
Hypovolemia increases the risk of nephrotoxic effects.
It is not recommended to use the solution as a means to prepare a patient for general anesthesia, as well as to maintain anesthesia during extensive surgical interventions.
During treatment with Ketorol, it is recommended to be careful when driving a car or using machinery.
gel (ointment) is recommended to be applied only to intact skin, avoiding contact with wounds and mucous membranes (including the eyes).
The drug should not be used under dressings made of airtight materials. After applying Ketorol to the skin, you should wash your hands well with soap.
Ketorol for acute back pain
Effective treatment of this group of patients implies timely relief of pain syndrome (PS), elimination of obstacles to carrying out the full scope of rehabilitation measures, and adequate expansion of the motor regime. It has been established that the earliest possible expansion of the motor mode (walking on a flat surface, performing familiar daily activities both at home and outside) is an important factor associated with early relief of pain and a reduction in disability. There is evidence that long-term bed rest (immobilization for more than 7 days), excessive restrictive behavior (both intuitively chosen by the patient himself and recommended by the doctor) are most closely associated with a high risk of increasing the duration of exacerbation, transformation of acute pain into chronic, high probability of developing depressive disorders [3]. Based on these considerations, it seems necessary to orient the patient toward the earliest possible inclusion in the program of restorative and rehabilitation measures, to recommend the patient active behavior as part of the course of restorative treatment, to form positive motivation that ensures the mood to achieve convalescence and compensation for the existing neurological and orthopedic defect. The patient himself should strive for the earliest possible return to the usual level of daily physical activity as the syndrome is relieved. Along the way, it should be noted that factors that negatively affect the effectiveness of therapeutic measures and the timing of rehabilitation therapy are the patient’s incomplete understanding of the cause and essence of his disease, reluctance to take an active position in achieving recovery, the expectation of a significant therapeutic effect from passive therapeutic measures (massage, physiotherapy and etc.). An important condition that determines the possibility of carrying out rehabilitation measures is the early and most complete elimination of BS. There is an obvious connection between the effectiveness of pain therapy and the duration of restoration of adequate motor activity. To relieve pain syndrome (PS), the most widely used drugs are from the group of nonsteroidal anti-inflammatory drugs (NSAIDs). One of the effective NSAIDs used incl. for the treatment of patients with dorsalgia, is Ketorol (ketorolac) - a derivative of arylacetic acid. In clinical practice, it is used in the form of tromethamine salt, which ensures high solubility of the drug in water. In accordance with the results of modern experimental and clinical studies, ketorolac is an effective inhibitor of cyclooxygenase types 1 and 2, which regulates the synthesis of prostaglandins, prostacyclin and thromboxane A2 from arachidonic acid. Ketorolac is a racemic mixture of S(-) and R(+) enantiomers, with the analgesic effect due to the S-form. Without interacting with opioid receptors, ketorolac does not have a cardiodepressive effect, does not depress respiration, and does not cause paresis of intestinal smooth muscles. Of undoubted interest are the recently presented results of experimental studies indicating that ketorolac has the ability to reduce the activation activity of spinal cord glial cells that provide pain sensitivity, in particular by inhibiting the expression of protease-activated receptor-1 [4]. As the authors of the study established, it was precisely due to these properties of the drug that its use significantly reduced the severity of allodynia if ketorolac was administered one day after the injury. It should be noted that the ability of ketorolac to eliminate allodynia caused in the primary headache model was established in another study [5]. The drug is characterized by high bioavailability (80–100%) and quickly enters the vascular bed both after oral and parenteral administration, which ensures rapid onset of analgesia. In practical terms, it should be borne in mind that food rich in fat increases the time to reach the maximum concentration of the drug in the blood by 1 hour. It has been established that after oral administration in a dose of 10 mg of the drug, the analgesic effect develops within 10–60 minutes, and after intramuscular administration in dose of 30 mg – after 15–75 minutes. In clinical settings, it has been demonstrated that the duration of action of ketorolac reaches 10 hours. Up to 95% of the drug entering the body binds to plasma proteins, which ensures the stability of the concentration of ketorolac in the blood. Ketorolac is metabolized primarily in the liver to form conjugated and hydroxylated forms, which are excreted by the kidneys. Given that a significant portion of the drug is excreted in the urine, impaired renal function is associated with the risk of developing toxic effects. The half-life of ketorolac in elderly patients is slightly longer than in young patients (4.7–8.6 and 3.8–6.3 hours, respectively). As noted above, ketorolac is well tolerated and has a low incidence of side effects (about 3%). The most common of these are dyspeptic disorders. Limiting the duration of treatment to achieve a positive effect can be a reliable way to increase the safety of therapy [6]. Currently, ketorolac is widely used in a number of countries to relieve acute BS caused by various pathological conditions, in particular to eliminate postoperative pain. A number of clinical studies have established the high analgesic effectiveness of the drug. The pronounced analgesic effect of ketorolac has attracted the attention of anesthesiologists and specialists faced with the need to relieve acute pain in somatic diseases and after surgical interventions. Thus, in one of the open pilot studies [7], intramuscular administration of ketorolac to 22 patients with severe postoperative PS provided an acceptable level of pain relief, and in 13 cases complete relief of pain was achieved. In 95% of patients, a decrease in pain intensity was observed during the first 60 minutes. after administration of the drug and increased over 2–4 hours, leading to complete elimination of pain in 50% of patients after 3.7 hours. The authors concluded that during the first hour after using ketorolac, additional administration of analgesic drugs is not advisable, given the high probability of delayed onset analgesic effect. An important property of ketorolac, in addition to pain relief, is its anti-inflammatory effect. It turned out that ketorolac administered into a postoperative wound in relatively small doses has an effect comparable to that after systemic use of NSAIDs, although the number of side effects turned out to be incomparably smaller [8]. This local use of the drug ensured a significant reduction in the need for patients to take opioid analgesics. According to the authors of the study, the effectiveness of the drug was due to the local anti-inflammatory effect, as evidenced by a decrease in the content of proinflammatory cytokines in the wound exudate. Similar data were obtained from another in vitro study. It turned out that ketorolac, introduced into cell culture, caused inhibition of the production of a number of proinflammatory cytokines and cell adhesion molecules, despite the fact that the effect was less pronounced than that of corticosteroids, it was significantly superior to that of placebo [9]. According to the authors, ketorolac has the ability to inhibit the cellular component of inflammation, which can find its practical application in the clinic. Somewhat later, a whole series of randomized, usually placebo-controlled, studies were conducted to evaluate the effectiveness of ketorolac in relieving acute BS caused by various pathological conditions. The objective of these works was to compare the effectiveness of ketorolac and representatives of other groups of analgesic drugs, in particular opioids and NSAIDs. In patients with BS caused by extensive surgery, intravenous administration of 30 mg of ketorolac in its analgesic activity was comparable to the administration of 4 mg of morphine [10]. Similarly, ketorolac was found to have comparable analgesic effects to another opioid derivative, meperidine (50 mg), when used in patients with acute hepatic colic, and the need for repeat analgesia was less likely to occur after ketorolac use [11]. The results of this work were confirmed somewhat later in a similar double-blind randomized study that included 324 patients with hepatic colic [12]. It turned out that 2 hours after the administration of ketorolac, the weakening of BS on the visual analogue scale (VAS) was 6.2±3.6 cm, and after the administration of meperidine – 6.7±3.6 cm (the differences were not significant; p=0 ,25). The authors noted better tolerability of ketorolac, as evidenced by a smaller number of patients who reported nausea, vomiting, and a feeling of general weakness. Another multicenter, double-blind study, including 125 patients undergoing arthroscopic autoplasty of the anterior cruciate ligament of the knee joint, compared the effectiveness of ketorolac (20 mg orally once) and a combination of 10 mg hydrocodone and 1000 mg paracetamol [13]. The effectiveness of ketorolac 1, 2 and 3 hours after administration was significantly higher, which allowed the authors to recommend the drug to eliminate BS in the postoperative period. Considering the analgesic effect and tolerability of ketorolac in comparison with opioids, it should be noted that there is experience in using the drug in pediatric practice: in children from 6 to 18 years old who have undergone orthopedic or arthrological surgery on large bones [14]. The results of an open study showed that the use of ketorolac (1.0 mg/kg parenterally as a loading dose, subsequently 0.5 mg/kg every 6 hours throughout the day) eliminates pain and significantly reduces the need for additional painkillers. Of interest are the results of a comparative study of the effectiveness of subacromial administration of triamcinolone or ketorolac for the purpose of relieving local BS [15]. Ketorolac has demonstrated its undoubted advantage as an analgesic agent with better tolerability. In a randomized, double-blind study that included 102 children, a single dose of ketorolac was not inferior to morphine in its analgesic effect [16]. It should be noted that in Russia the drug is approved for medical use in patients at least 16 years of age. Almost all researchers who studied the analgesic effectiveness of ketorolac noted its good tolerability. The undoubted advantage of ketorolac compared to narcotic painkillers is the absence of an inhibitory effect on respiratory function and the cardiovascular system, as well as the absence of sedation. It is important to note that there is no risk of addiction. It is also necessary to mention the absence of gastrotoxicity during short courses of treatment with ketorolac in order to eliminate acute BS. Considering the effectiveness of ketorolac in relieving postoperative pain, attempts have been made to use it in patients with acute pain caused by degenerative lesions of the articular-ligamentous apparatus. A randomized, double-blind study compared the effectiveness of intramuscular meperidine (1 mg/kg) and ketorolac (60 mg) for the relief of acute musculoskeletal pain in the low back in 155 patients over the age of 18 years [17]. The analgesic effect of the drugs according to VAS was comparable: a 30% decrease in pain intensity was recorded in 63% of patients receiving ketorolac and 67% of patients in the meperidine group; 35% of patients who received ketorolac and 37% who received meperidine required additional painkillers. The authors note that, with a comparable analgesic effect, treatment tolerability was significantly better in patients receiving ketorolac; they experienced significantly less unwanted side effects (nausea, repeated vomiting, drowsiness). A large-scale study was conducted in Moscow to study the effectiveness of ketorolac in patients with acute BS caused by inflammatory diseases of the musculoskeletal system, degenerative diseases of the spine (arthralgia, lumbago, ischalgia) and some other pathological conditions [18]. The study was conducted in the setting of prehospital medical care in an outpatient setting. A total of 1011 patients (mean age 54.1±0.46 years) who received ketorolac (30 mg intramuscularly) or other analgesic drugs, in particular metamizole sodium (2 ml of 50% solution intramuscularly) took part in the study. According to the results of assessing the dynamics of pain using VAS, the analgesic effect of ketorolac is 1.3 times greater than that of metamizole sodium. It is significant that the need to repeatedly seek medical help (call an ambulance team) for musculoskeletal pain was 3 times higher in patients who received metamizole (18% versus 6.2%). The onset of the analgesic effect after the administration of ketorolac was observed after an average of 12.4±0.33 minutes, whereas after the use of metamizole - after 26.9±0.47 minutes. (the difference was significant), and the severity and speed of onset of the effect did not significantly depend on the age of the patients. The data obtained allowed the authors to conclude that the use of ketorolac in the prehospital stage of treatment of acute BS of various etiologies is effective and clinically justified in various pathological conditions, in particular in acute musculoskeletal pain. It should be noted that, according to the results of the cited study, the use of ketorolac has certain pharmacoeconomic advantages: the value of such a parameter as the cost per unit of effectiveness turned out to be 3 times less than with the use of metamizole (the differences were significant), and significantly lower than with use of other NSAIDs. Almost simultaneously, an open, uncontrolled clinical trial was conducted, the purpose of which was to evaluate the effectiveness and safety of the use of parenteral and oral forms of ketorolac (Ketorol) in relieving moderate and severe back pain (at least 940 mm on VAS) [18]. The study included 30 patients aged 30–65 years (average age: 49.4 years, 17 men) with degenerative lesions of the lumbar spine. Diagnoses were verified based on the results of clinical and radiological studies. The duration of treatment for patients did not exceed 5 days. At the beginning of therapy, ketorolac was prescribed as intramuscular injections of 60 mg/day. (30 mg 2 times/day) for 2 days, and then in tablet form 20 mg/day. During the study period, the use of other NSAIDs was excluded. During the study, it was found that as a result of a 5-day course of using ketorolac, the intensity of BS according to VAS significantly decreased, and after parenteral administration of the first dose of the drug it decreased from 65.4 to 22.1 mm (p < 0.05). At the same time, the volume of active movements in the lumbar spine increased, which was reflected in the results of the Thomaier tests (increase in the range of movements by 33% from the initial indicators) and Schober (increase by 25%). Based on the data obtained, the authors concluded that ketorolac is highly effective and well tolerated. It seems important that when assessing the safety of the drug, only 16% of patients had side effects (gastralgia, feeling of fullness in the stomach, headache accompanied by a rise in blood pressure). These side effects were not severe and did not require discontinuation of the drug or change in its dosage regimen. Over the past years, significant experience has been accumulated in the use of ketorolac not only in patients with musculoskeletal pain syndromes, but also in other pathological conditions in neurology associated with intense pain. Of undoubted interest is the possibility of using the drug to relieve an acute attack of migraine. The results of meta -analysis of 8 randomized clinical studies (321 patients are included, of which 141 received ketorol parenterally), selected from 32 dedicated to the possibility of its use in migraine attack, showed that the drug is able to effectively stop the hemicacular attack [20]. It turned out that, by the ability to eliminate the attack of Ketorolak pain, he exceeded the constituent, introduced by orally, while its use was not associated with the risk of dependence, which could be observed when using some opioids, in particular meteridine. Thus, the results of numerous studies indicate that ketorolac (ketorol) is an effective and safe drug for the treatment of acute pain in the lower back. The use of Ketorolak (ketorol) in such patients in its effectiveness not only surpasses many NSAIDs, but is not inferior to weak opioids. Subject to the reception regimen, the drug is well tolerated by patients and does not cause serious side effects. Literature 1. Carey T., Evans A., Hadler N. et al. Acute Severe Low Back Pain. APPULATION-BASED Study of Prevalence and Care-seeking // Spine. 1996. Vol. 21. P. 339–344. 2. Luo X., Pietrobon R., Sun S. et al. Estimates and Patterns of Direct Health Care Expenditures Among Individuals with Back Pain in the United States // Spine. 2004. Vol. 29. P. 79–86. 3. Bair M., Robinson R., Katon W. et al. Depression and Pain Comorbidity: A Literature Review // Arch. Intern. Med. 2003. Vol. 163. P. 2433–2445. 4. Dong L., Smith J., Winkelstein B. Ketorolac Reduces Spinal Astrocytic Activation and Par1 Express Associated with AttenUtion of Pain Following Following Joint Injury // J. NeUROTRAMA. 2012. 5. Oshinsky M., Sangvi M., Maxwell C. et al. Spontaneous Trigeminal Allodynia in Rats: A Model of Primary Headache // Headache. 2012. Vol. 52 (9). P. 1336–1349. 6. BJarnason I. GastroinTestinal Safety of Nsaids and Over-the-Counter Anaalgesics // Int. J. Clin. Pract. Suppl. 2013. Vol. 178. P. 37–42. 7. Perez-Urizar J., Granados-Soto V., Castaneda-Hernandez G. et al. Anaalgesic Efficacy and Bioavailability of Ketorolac in Postoperation Pain: A Probability Analysis // Arch. Med. Res. 2000. Vol. 31 (2). P. 191–196. 8. Carvalho B., Lemmens H., Ting V., Angst M. Postoperation Subcutaneous Instillation of Low-Dose Ketorolac But Hydromorphone Reduces Wound Concentrations of Intereleum 6 and InterleUKIN-10 and IMPROVES ANALGESI Following Cesarean Delivery // J. Pain. 2013. Vol. 1. P. 48–56. 9. Mazzocca A., McCarthy M., Intravia J. et al. An in vitro evaluation of the anti-inflamMatory Effects of Platelet-Rich Plasma, Ketorolac, and Methylprednisolone // Arthroscopy. 2013. PII: S0749–8063 (12) 01896–8. DOI: 10.1016/J.Arthro 2012.12.005. 10. Brown C., Moodie J., Wild V. et al. Comparison of Intravenous Ketorolac Tromethamine and Morphine Sulfate in the Treatment of Postoperation Pain // PharmacoPyrapy. 1990. Vol.10 (6 (PT 2)). 116S --121S. 11. Dula D., Anderson R., Wood G. A Prospective Study Comparing Im Ketorolac with Im Meperidine in the Treatment of Acute Biliary Colic // J. Emerg. Med. 2001. Vol. 20 (2). P. 121–124. 12. Henderson S., Swadron S., Newton E. Comparison of Intravenous Ketorolac and Meperidine in the Treatment of Biliary Colic // J. Emerg. Med. 2002. Vol. 23 (3). P. 237–241. 13. Barber F., Gladu D. Comparison of Oral Ketorolac and Hydrocodone for Pain Relief After Anterior Cruciate Reconstruction // Arthroscopy. 1998. Vol. 14 (6). P. 605–612. 14. EBERSON C., Pacicca D., Ehrlich M. The Role of Ketorolac in Decreasing Length of Stay and Narcetic Complications in the Postepection Pediatric Orthopaedic Patient // J. Pedicatr. Orthop. 1999. Vol. 19 (5). P. 688–692. 15. Min K., Pierre P., Ryan P. et al. Elbow. Surg. 2012. PII: S1058–2746 (12) 00372–2. Doi: 10.1016/J.JSE. 2012.08.026. . 16. Lieh-lai M., Kauffman R., Uy H. et al. A RANDOMIZED Comparison of Ketorolac Tromethamine and Morphine for Postoperation Anaalgesia in Critically Ill Children // Crit. Care Med. 1999. Vol. 27 (12). P. 2786–2791. 17. Veenema K., Leahey N., Schneider S. Ketorolac Versus Meperidine: Ed Treatment of Severe Musculoskeletal Low Back Pain // Am. J. Emerg. Med. 2000. Vol. 18 (4). P. 40404–40407. 18. Vertkin A.L., Prokhorovich E.A., Goruleva E.A. and others. The effectiveness and safety of the use of ketorol to stop the pain syndrome at the prehospital stage // Emergency therapy. 2004. No. 1-2; 16–17: 12–17. 19. Shostak N.A., Pravyuk N.G., Aksenova A.V. and other possibilities of optimizing analgesic and anti -inflammatory therapy in patients with acute pain in the back // breast cancer. 2006. No. 14; 8. S. 610–615. 20. Taggart E., Dran S., Kokotillo A. et al. Ketorolac in the Treatment of Acute Migraine: A Systematic Review // Headache. 2013. Vol. 53(2). P. 277–287.
Ketorol's analogs
Level 4 ATC code matches:
Voltaren Emulgel
Ultrafastin
Indomethacin
Dicloran
Dicloran Plus
Dolgit
Nise
Ketonal
Febrofeed
Fastum gel
Diclofenac
Finalgel
Bystrumgel
Deep Relief
Butadion
Diklovit
Artrosilene
Olfen
Fanigan Fast
Structural analogues of tablets and solution: Adolor , Vatorlak , Ketanov , Dolak , Dolomin , Ketofril , Ketalgin , Ketorolac , Ketocam .
Drugs with a similar mechanism of action: Arthrotek , Bioran , Voltaren , Diclak , Diclogen , Diclofenac , Indomethacin , Naklofen , Ortofen , Neurodiclovit , Flotac , SwissJet .
Suppositories with a similar effect: Voltaren , Diclak , Diclonate P , Naklofen , Diclofenac , Indomethacin .
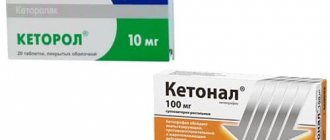
What is better - Ketorol or Ketonal?
Ketonal is a drug based on the NSAID ketoprofen (a derivative of propionic acid). The medicine has the same indications for use as Ketorol.
When administered parenterally, the analgesic effect appears within 15-30 minutes. With intravenous infusion of ketorolac , plasma concentrations reach maximum values after 4 minutes.
The difference between ketoprofen and ketorolac is also a shorter half-life - less than 2 hours.
Studies of the effectiveness of drugs for pain relief in postoperative patients have shown that ketorolac provides a faster, more effective and longer-lasting effect than its analogue, and also has a lesser effect on the hemostatic system.
Ketorol injection solution 30mg/ml 1ml amp 10 pcs
Pharmacological group:
Non-steroidal anti-inflammatory drug.
Pharmacodynamics:
Non-steroidal anti-inflammatory drug (NSAID), has a pronounced analgesic effect, has anti-inflammatory and moderate antipyretic effects. The mechanism of action is associated with non-selective inhibition of the activity of cyclooxygenase (COX) - COX-1 and COX-2, which catalyzes the formation of prostaglandins from arachidonic acid, which play an important role in the pathogenesis of pain, inflammation and fever. Ketorolac is a racemic mixture of [-]S and [+]R enantiomers, and the analgesic effect is due to the [-]S form. The strength of the analgesic effect is comparable to morphine, significantly superior to other NSAIDs.
The drug does not affect opioid receptors, does not depress respiration, does not cause drug dependence, and does not have a sedative or anxiolytic effect.
Pharmacokinetics:
The pharmacokinetics of ketorolac after single and repeated intravenous and intramuscular administration is linear.
When administered intramuscularly, absorption is complete and rapid. The maximum concentration of the drug (Cmax) after intramuscular administration of 30 mg is 1.74-3.1 μg/ml, 60 mg is 3.23-5.77 μg/ml, time to reach maximum concentration (TCmax) is 15-73 min and 30-60 minutes, respectively. Cmax after intravenous administration of 15 mg – 1.96-2.98 mcg/ml, 30 mg – 3.69-5.61 mcg/ml, TCmax – 0.4-1.8 min and 1.1-4.7 min, respectively. Communication with plasma proteins – 99%. The time to reach the equilibrium concentration of the drug (Css) with parenteral administration of 30 mg 4 times a day is 24 hours; with intramuscular administration, 15 mg – 0.65–1.13 µg/ml, 30 mg – 1.29-2.47 µg/ml.
The volume of distribution (Vd) with intramuscular administration is 0.136-0.214 l/kg, with intravenous administration - 0.166-0.254 l/kg. In patients with renal failure, the volume of distribution of the drug may double, and the volume of distribution of its R-enantiomer may increase by 20%.
Penetrates into breast milk: when the mother takes 10 mg of ketorolac, Cmax in milk is achieved 2 hours after taking the first dose and is 7.3 ng/ml, 2 hours after taking the second dose of ketorolac (when using the drug 4 times a day) - 7 .9 ng/l. About 10% of ketorolac passes through the placenta.
More than 50% of the administered dose is metabolized in the liver with the formation of pharmacologically inactive metabolites. The main metabolites are glucuronides, which are excreted by the kidneys, and the pharmacologically inactive p-hydroxyketorolac. It is excreted 91% by the kidneys, 6% through the intestines.
The half-life (T1/2) in patients with normal renal function is 3.5-9.2 hours after parenteral administration of 30 mg. T1/2 increases in elderly patients and shortens in young ones. Changes in liver function do not affect T1/2. In patients with impaired renal function, with a plasma creatinine concentration of 19-50 mg/l (168-442 µmol/l), T1/2 - 10.3-10.8 hours, with more severe renal failure - more than 13.6 h.
When 30 mg of ketorolac is administered intramuscularly, the total clearance is 0.023 l/h/kg (0.019 l/h/kg in elderly patients); in patients with renal failure (with a plasma creatinine concentration of 19-50 mg/l) – 0.015 l/h/kg. When 30 mg of ketorolac is administered intravenously, the total clearance is 0.03 l/h/kg.
Not excreted by hemodialysis.
Is it possible to give Ketorol to children?
The annotation states that all dosage forms of the drug are intended for the treatment of patients over 16 years of age (according to Wikipedia, age under 16 years of age is a relative contraindication).
The reason for this restriction is that the use of ketorolac in children can cause visual and hearing impairment, depression , nephritis , pulmonary edema , allergic reactions and other severe complications.
Therefore, it is better to give the child safer means to relieve pain - for example, paracetamol or ibuprofen .
Reviews of Ketorol
Reviews of Ketorol tablets, as well as reviews of injections and gel, are positive in 95-98% of cases. Ketorol helps with toothache, back and muscle pain, and headaches within half an hour, and its effect lasts up to six hours.
Most patients note that when using the medicine in accordance with the instructions, side effects occur extremely rarely. Sometimes the disadvantages of Ketorol are a large number of contraindications and the impossibility of long-term use.
How much do Ketorol tablets, gel and ampoules cost?
The price of Ketorol in tablets in Ukrainian pharmacies is 29-37 UAH. Injections can be bought for 98-105 UAH. The price of Ketorol gel is 55-62 UAH.
In Russia, the average price of Ketorol in tablets is 45 rubles, the price of Ketorol in ampoules is 145 rubles, the cost of ointment (gel) is 215 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Ketorolac tab.
p/o captivity. 0.01g 14pcs ZAO PFK Update RUB 39.order - Ketorolac tab. p/o captivity. 0.01g 28pcs JSC PFK Renewal
69 RUR order
- Ketorolac tab. p.p.o. 10mg 20pcs JSC Tatkhimfarmpreparaty
35 rub. order
- Ketorolac solution for intravenous and intramuscular administration. 30mg/ml amp. 1ml 10 pcs. Ozone LLC
86 rub. order
- Ketorol solution for intravenous and intramuscular administration. 30mg/ml 1ml 10 pcs.Dr. Reddy's lab.
134 RUR order
Pharmacy Dialogue
- Ketorol (gel tube 2% 50g)Dr. Reddy's
RUB 374 order
- Ketorolac (amp. 30 mg/ml 1 ml No. 10) Sintez (Kurgan) OJSC
84 rub. order
- Ketorolac (amp. 30 mg/ml 1 ml 2x5 No. 10) Ellara LLC
54 RUR order
- Ketorolac (amp. 30 mg/ml 1 ml No. 10) Welfarm LLC
55 rub. order
- Ketorol (gel tube 2% 30g)Dr. Reddy's
RUB 262 order
show more
Pharmacy24
- Ketorol 2% 30 g gel Dr. Reddy's Laboratories Ltd., India
72 UAH.order - Ketorol 30 mg/1 ml N10 solution Dr. Reddy's Laboratories Ltd., India
175 UAH order
- Ketorol 10 mg No. 20 tablets Dr. Reddy's Laboratories Ltd., India
75 UAH order
PaniPharmacy
- Ketorol tablets Ketorol film-coated tablets 10 mg No. 20 India, Dr. Reddy's
93 UAH order
- KETOROLAC ampoule Ketorolac r/r d/in. 3% 1ml. No. 10 Ukraine, Health LLC
99 UAH order
- Ketorol ampoule Ketorol solution d/in. 30mg amp. 1ml No. 10 India, Dr. Reddy's
152 UAH order
- KETOROLAC tablets Ketorolac tablets. 10mg No. 10 Ukraine, Health LLC
18 UAH order
- Ketorol gel Ketorol gel 2% 30g India, Dr. Reddy's
85 UAH order
show more