From this article you will learn:
- consequences of fluoride deficiency in the body,
- Why is fluoride important for teeth?
- best fluoride toothpaste,
- risks of developing fluorosis in children.
The article was written by a dentist with more than 19 years of experience.
Fluorine is a chemical element of the halogen group, which under normal conditions is a diatomic gas. Fluorine is a strong oxidizing agent, toxic in high concentrations, but nevertheless it is a vital element for the body. In the human body it is found mainly in bones and tooth enamel. The total mass of fluoride in the body of an adult is about 2.6 g (of which 2.5 g is in the bones). The main property of fluoride is that it prevents the “washing out” of calcium from bone tissue and tooth enamel.
The normal daily intake of fluoride into the human body is from 2.5 to 3.5 mg. Insufficient consumption of fluoride in drinking water (less than 0.5 mg per liter) sharply increases the risk of developing dental caries. Fluoride is one of the most important components of tooth enamel. Fluoride ions, binding to the surface layer of enamel, give it high strength and resistance to destruction by oral bacteria. Research shows that with regular use of fluoride toothpastes, the incidence of dental caries is reduced by up to 70%.
The best pastes with fluoride (from the author of the article) –
Thus, the anti-caries activity of fluoride is very important for keeping human teeth healthy. Fluoride in toothpaste has been used for 100 years, and fluoridation of drinking water has been used since the 1950s. In the production of toothpastes, the following 4 types of fluoride compounds are usually used: sodium fluoride, aminofluoride (synonym - olaflur), tin fluoride and, most rarely, sodium monofluorophosphate. And below we will talk about all these connections.
Moreover, in the composition of such compounds, fluorine is initially inactive. And only after the toothpaste has entered the oral cavity (at body temperature and the presence of saliva) do these compounds begin to dissociate into ions. This is how active fluoride ions appear, which are embedded in tooth enamel. At the same time, fluoride ions are not able to penetrate the mucous membrane of the mouth and be absorbed into the blood - this can only happen when swallowing toothpaste.
Long-term fluoride deficiency can lead to the development of -
For adults, the optimal fluoride intake is about 4 mg per day, and the upper limits of acceptable intake are up to 10 mg per day. For children, the optimal intake will be from 0.01 to 1 mg per day (depending on gender and age) - you will see more accurate numbers in the table below. Daily sources of fluoride for humans are water and food. Fluoride-rich foods include fish, walnuts, and some dairy products.
With food, a person should receive between 0.25 mg and 0.35 mg of fluoride, and with water, usually about 1.0-1.5 mg of fluoride per day (assuming you drink 1 to 1.5 liters of water per day). day, and also live in a region where there is no shortage of fluoride in drinking water). The norm for fluoride content in drinking water is from 0.7 to 1.0 mg/l. An excess of fluoride concentration in drinking water is observed only in 21 regions of Russia, and in most regions there is a lack of it (in some regions it is below 0.1 mg/l). Therefore, most people do not get the required amount.
Regions with high fluoride content –
- The Moscow, Tver, Ryazan, Sverdlovsk, and Chelyabinsk regions have an increase in the fluorine content of natural origin (in addition, in the Ryazan and Sverdlovsk regions, very large coal-fired thermal power plants additionally increase the level of fluorine in water).
- In the Kemerovo, Irkutsk, and Sverdlovsk regions, as well as in the Krasnoyarsk Territory and the Republic of Khakassia, the increase in fluoride in drinking water is associated with the presence of aluminum and cryolite production plants.
- In the Vladimir, Moscow, Volgograd, Ryazan regions, Udmurtia and Mordovia, the increase in fluoride in drinking water is associated with the presence of glass factories.
- In the Kostroma and Kirov regions, the increase in fluoride content in water is associated with factories for the production of organic phosphorus fertilizers.
Important: please note that if you use filtered drinking water, then osmotic filters reduce the fluoride content in water by approximately 84%, and carbon filters - by at least 81% (this was proven by research by Brown MD and Aaron G., publication in the journal "Pediatr.Dent" for 1991). Therefore, even if you live in a region with a high level of fluoride in water, your fluoride consumption can be within normal limits, simply if you regularly change the filter cartridges.
If there is insufficient fluoride in the body, the following develops:
We have already said that the body of an adult contains approximately 2.6 g of fluoride, and 2.5 g of this is contained in bone tissue. Research confirms that insufficient fluoride intake contributes to decreased bone mass as well as increased bone fragility. In particular, a lack of fluoride leads to impaired saturation of bone tissue with phosphates. That is why sodium fluoride tablets (for oral administration) are often prescribed for osteoporosis - this allows for an increase in bone mineral density.
A long-term lack of fluoride can lead to the development of dental caries and impaired mineralization of teeth in children and adolescents (24stoma.ru). Comparative studies of the use of toothpastes with and without fluoride have shown that if you refuse to use fluoride toothpastes, the increase in caries occurs at least 35-40% faster.
Fluoride intake standards for children:
The US National Academy of Sciences, as well as the US Environmental Protection Agency (USEPA), have recommended the following doses of fluoride intake, which are considered safe for health in general and for the development of fluorosis in particular.
Table 1 is the recommended total daily fluoride intake for children 0 to 19 years of age. Link to scientific publication (Source)
In this guideline, the suggested total daily intake for children 0 to 6 months of age is 0.01 mg fluoride per day in all beverages and foods. This means you must account for all of your baby's water intake (including that you use to dilute formula), as well as that contained in other foods or supplements.
How much fluoride does a person need and what foods contain it?
The daily dose of fluoride required for an adult is 0.03 mg/kg. During the period of formation and growth of the skeleton, the child’s body needs a larger amount - 0.15-0.1 mg/kg.
You can get this microelement from foods that are present in your daily diet:
- fish (the best source is mackerel, pollock, cod);
- all types of meat and offal;
- dairy products;
- raw nuts: walnuts, cashews, hazelnuts;
- fruits and vegetables (pumpkin, grapefruit, apple);
- mushrooms (ceps, chanterelles).
A balanced diet allows you to get fluoride from foods that are always in the refrigerator.
History of the use of fluoride in toothpaste -
Fluoride was first added to Tanagra toothpaste in 1890, but it was not widely used. In the 1940s, a second wave of fluoride toothpastes began appearing in the United States, but their use was initially criticized by the American Dental Association (ADA). Only after numerous clinical studies were carried out, in 1950, ADA approval for their industrial production was received.
In parallel with this, in 1945 the technology of systemic fluoridation of drinking water was first used in the USA. Initially, this was implemented in 4 American cities (Evanston, Newberg, Grand Rapids and Brantford), where 1 mg of fluoride per 1 liter of water was added to drinking water at treatment stations. As a result, a reduction in the incidence of caries in the population by approximately 50% was achieved.
Thus, the first toothpaste with fluoride, which was clinically proven effective against caries, was released by Procter & Gamble under the Crest brand (USA). And after only 5 years of observation, dental associations from different countries recognized the high anti-caries effectiveness of pastes with fluoride (subject to their regular use for oral hygiene).
Clinical researches
Clinical studies have proven that regular use of professional toothpaste ASEPTA REMINERALIZATION improved the condition of the enamel by 64% and reduced tooth sensitivity by 66% after just 4 weeks.
Sources:
- Report on the determination/confirmation of the preventive properties of personal oral hygiene products “ASEPTA PLUS” Remineralization doctor-researcher A.A. Leontyev, head Department of Preventive Dentistry, Doctor of Medical Sciences, Professor S.B. Ulitovsky First St. Petersburg State Medical University named after. acad. I.P. Pavlova, Department of Preventive Dentistry
- Study of the clinical effectiveness of treatment and prophylactic agents of the Asepta line in the treatment of inflammatory periodontal diseases (A.I. Grudyanov, I.Yu. Aleksandrovskaya, V.Yu. Korzunina) A.I. GRUDYANOV, Doctor of Medical Sciences, Prof., Head of Department I.Yu. ALEXANDROVSKAYA, Ph.D. V.Yu. KORZUNINA, asp. Department of Periodontology, Central Research Institute of Dentistry and Maxillofacial Surgery, Rosmedtekhnologii, Moscow
- Clinical studies of antisensitive toothpaste “Asepta Sensitive” (A.A. Leontyev, O.V. Kalinina, S.B. Ulitovsky) A.A. LEONTIEV, dentist O.V. KALININA, dentist S.B. ULITOVSKY, Doctor of Medical Sciences, Prof. Department of Therapeutic Dentistry, St. Petersburg State Medical University named after. acad. I.P. Pavlova
What is the anti-caries effect of fluoride associated with:
The anti-carious effect of fluoride is multifaceted, but the most important is its regulating effect on the processes of demineralization and remineralization of tooth enamel. And we will talk about this in more detail below.
Inclusion of fluorine in the composition of tooth enamel apatites –
Tooth enamel consists of hydroxyapatite crystals (Ca10(PO4)6OH2). They begin to break down when exposed to organic acids, which are produced by cariogenic bacteria in the oral cavity. Bacteria “digest” food debris in the mouth, releasing lactic, acetic and propionic acids, which begin to dissolve tooth enamel. When there is enough acid in the oral fluid and the pH of the oral fluid drops below 5.5, the process of demineralization of the enamel begins.
In this case, hydroxyapatite crystals begin to dissolve, i.e. the enamel begins to lose Ca2+, PO43- (calcium and phosphate ions). For enamel hydroxyapatite, the critical level is pH 5.5 - below which demineralization always occurs. But when using fluoride toothpaste or professional fluoridation of teeth, fluoride ions (F−) can be included in the structure of hydroxyapatite crystals, replacing hydroxide ions (OH−). As a result, apatites such as fluorohydroxyapatite and fluorapatite are formed:
Ca10(PO4)6OH2 ⇔ Ca10(PO4)6(OH F) or Ca10(PO4)6F2
Apatite crystals with fluorine ions included in them are much more resistant to organic acids, and their dissolution begins not at pH 5.5, but at a more acidic pH of 4.5. Accordingly, the first mechanism of the anti-caries effect of fluoride (discussed in this section) is that fluorides make the enamel more resistant to the effects of organic acids produced by cariogenic bacteria in the oral cavity.
Important: but it should be noted that the formation of fluorapatites, although a very important mechanism for the anti-carious action of fluorides, still has significantly lower anti-carious effectiveness - in comparison with the importance of the formation of a layer of calcium fluoride on the enamel surface.
Formation of a layer of calcium fluoride on the surface of the enamel -
But the anti-caries effect of fluorine is not limited to the formation of fluorinated apatites. Fluoride ions are able to enter into chemical reactions with calcium and phosphate ions (Ca2+, PO43-) - both contained in saliva and those formed as a result of the dissolution of enamel by acid. As a result of these chemical reactions, a protective layer is formed on the surface of the enamel, consisting of globules of calcium fluoride (CaF2). Calcium fluoride is a slightly soluble compound in water, but its solubility increases sharply when exposed to acid - if the pH reaches 5.0 or lower.
The anticarious effect of calcium fluoride is realized as follows. Firstly, the CaF2 layer protects the surface of the teeth from direct acid attack, i.e. it represents the so-called “sacrificial layer”. Secondly, in an acidic environment (i.e. during an acid attack), calcium fluoride globules begin to dissolve, which leads to the release of active calcium and fluorine ions. Those. The CaF2 layer on the surface of the enamel is not only a sacrificial layer, but also a kind of depot of calcium and fluorine ions.
Important: Scientific studies (including Ogaard et al., 1988) have found that small amounts of free fluoride ions in the oral fluid surrounding the enamel slow down the acid dissolution of enamel more effectively than the fluoride ions found in tooth enamel. It is the formation of the CaF2 layer on the surface of the enamel that makes it possible to maintain high concentrations of fluoride and calcium ions in the oral fluid, and in fact is the main element of fluoride anti-caries protection.
How long does the layer of calcium fluoride last? With daily use of fluoride toothpastes and rinses, the layer of calcium fluoride will be replenished daily, and especially thick layers are formed precisely over areas of demineralization of tooth enamel (to a lesser extent – over healthy enamel). Moreover, in studies (Klimek et al., 1998) it was found that calcium fluoride is significantly deposited on tooth enamel - specifically when using toothpastes with amino fluoride , and to a much lesser extent when using toothpastes with sodium fluoride.
But when using highly concentrated fluoride preparations in a dental office, a significant layer of calcium fluoride is formed (both when using amino fluoride and sodium fluoride). Most of this layer is lost in the first hours or days - first of all, it disappears on the chewing surfaces of the teeth as a result of abrasion, but it persists for quite a long time, primarily in areas of increased risk of caries - on contact surfaces, in fissures, as well as on the surface of lesions demineralization of enamel.
A scientific work (Caslavska et al., 1991) revealed that when a layer of calcium fluoride was formed precisely over the foci of enamel demineralization, a significant amount of it was still detectable even after 6 weeks, and an insignificant amount even after 18 months. In in situ studies (Attin et al, 1995), after a single local application of a concentrated fluoride preparation, a loss of 80% of calcium fluoride was observed after 5 days. In all these and other studies, it was simultaneously revealed that the loss of calcium fluoride occurred with a simultaneous increase in the fluoride content in the enamel structure, i.e. fluorapatites increased (Hellwig et al., 1989; Buchalla et al., 2002).
An interesting point: the mechanism of “fluoridation of apatites” and the mechanism of “formation of a layer of calcium fluoride” are involved both when using the most common oral hygiene products for home use, and when using highly concentrated fluorides by a dentist. But less concentrated home remedies will work mainly due to the “fluoridation of apatites”, and when using highly concentrated fluorides, the process of calcium fluoride formation is more pronounced.
Fluoride is a catalyst for the remineralization of teeth -
This interesting feature of fluorine was discovered relatively recently. It turned out that fluorine stimulates so-called phase transformations in minerals, which is manifested by accelerated growth of hydroxyapatite crystals in tooth enamel. Those. In the presence of fluoride ions, enamel remineralization always occurs faster. That is why the best products for remineralizing teeth contain not only sources of calcium and phosphates, but also fluorides (for example, the “GC MI Paste Plus” gel contains CPP-ACP + 900 ppm sodium fluoride).
Important: but that's not all. The presence of fluoride ions in the oral fluid ensures the remineralization of teeth even at a more acidic pH of 5.5-4.5 - when normally, on the contrary, demineralization of the enamel should already occur. This is a complex mechanism that we will not dwell on, but it is also due to the fact that fluorine ions (F−) are able to replace hydroxide ions (OH−) in the structure of hydroxyapatite crystals.
Other mechanisms of the anti-caries effect of fluoride -
The most important anti-caries properties of fluoride are, of course, associated with its influence on the processes of demineralization and remineralization of enamel - and we described this in detail above. But the effects of fluoride don’t stop there:
- Fluorides (especially amino fluoride) are capable of saturating microbial plaque and also attaching to the oral mucosa for a long time. This creates a depot of fluoride ions - similar to the depot that creates a layer of calcium fluoride on the surface of the enamel, and has a truly important anti-caries value.
- Fluoride has a bactericidal effect on dental plaque microorganisms - due to the inhibition of intracellular enzymes (in fact, fluorides are natural antiseptics that reduce the rate of proliferation of cariogenic bacteria in the oral cavity).
- Fluorides can suppress the acid-forming activity of cariogenic bacteria.
- Fluorides prevent microbial plaque from attaching to the enamel surface, i.e. prevent the formation of dental plaque. This property is realized by disrupting the formation of extracellular polysaccharides by bacteria.
The anti-caries effectiveness of fluoride products depends on a number of factors:
- on the type of fluoride chemical compound in the toothpaste,
- fluoride concentration (ppm),
- frequency of use of fluoride paste,
- type of product (rinse, paste, gel or varnish),
- the duration of contact of fluoride ions with the enamel (there are differences in the rate of dissociation of different fluoride compounds into active ions, but this will also depend on the time of brushing the teeth and on the form of the drug used).
Elevated levels of fluoride in the oral cavity - can persist from 2 hours to several weeks, depending on the form of fluoride-containing product used and the type of fluoride compound. Therefore, the frequency of use of fluoride-containing products is determined by the rate at which the effect disappears after a single use, and can vary from 3 times a day to 1-2 times a year. The duration of contact of fluoride with enamel increases in the following order: rinse → toothpaste → gel → varnish with fluoride.
Fluoride-free products for children
For children under 3 years of age, special pastes and gels are produced that do not contain fluorides, bleaching or aggressive substances. As a rule, natural ingredients are included in children's hygiene and preventive products.
Popular fluoride-free baby toothpastes:
| Product name | Country of Origin | Main components | Short description |
| Weleda | Germany |
| The gel has an anti-inflammatory effect and effectively removes plaque from teeth. Thanks to its completely natural composition, it is completely safe for the health of a small child. |
| President Baby | Italy |
| Toothpaste cleanses the oral cavity well of plaque and harmful microorganisms. |
| Rocs Baby | Russian Federation |
| The paste effectively fights the inflammatory process. However, it does not have an anti-caries effect. |
Sodium monofluorophosphate –
Sodium monofluorophosphate (Na2PFO3) is currently used only in very inexpensive toothpastes. The dissociation of this compound into ions (with the release of active fluorine) occurs very slowly, and this process also requires an oral enzyme such as phosphatase. Therefore, the level of fluoride ions will depend not only on the concentration of monofluorophosphate in the toothpaste, but also on the activity of phosphatase (this level is different for each person).
Moreover, such a component of toothpastes as sodium lauryl sulfate has the property of suppressing the activity of phosphatase in the oral cavity, which leads to an even greater reduction in the caries-preventive effect when using monofluorophosphate. In addition, sodium monofluorophosphate either does not lead to the formation of any significant layer of calcium fluoride on the surface of the teeth (later this layer should become a source of fluorine and calcium for the enamel), or its amount was close to zero. The only advantage of this compound is that it can be combined with calcium-based abrasives, because this does not lead to a decrease in the concentration of active fluorine ions.
The critical disadvantages that do not allow the use of monofluorophosphate for caries prevention are 1) too long a time for its dissociation into active ions, 2) lack of formation of calcium fluoride. For example, if you only brush your teeth for 3 minutes or less, this fluoride will be completely ineffective. But even if you brush your teeth for more than 3 minutes, then using toothpaste with monofluorophosphate will in any case not lead to the deposition of any significant layer of calcium fluoride on the surface of the enamel (as a depot of calcium and phosphates).
Is there an alternative?
With the right approach and judicious use, fluoride is an effective way to treat and prevent tooth sensitivity, caries and enamel demineralization. But, if you still have doubts, there is an alternative - pastes with synthetic hydroxyapatite.
This hydroxyapatite is completely identical to natural hydroxyapatite, the one that makes up tooth enamel. The substance is easily integrated into the crystal lattice of the enamel, strengthening it, reducing sensitivity and preventing the development of caries. The only drawback is that pastes with hydroxyapatite are more expensive than fluoride-containing ones.
Sodium fluoride –
Sodium fluoride (NaF) is the most common fluoride compound - both in toothpastes and preparations for professional fluoridation of teeth at the dentist (the only question is the difference in concentrations). Sodium fluoride, entering the oral cavity, quite easily dissociates into ions with the release of active fluoride ions. This usually takes about 30-40 seconds. In addition, it adheres well not only to tooth enamel, but also to plaque and the oral mucosa.
Sodium fluoride has a moderate remineralizing effect and a pronounced caries-preventive effect. A single treatment of teeth with fluoride varnish (once every 3-6 months) - when using a product with 1% sodium fluoride, reduces the risk of developing dental caries by up to 30%, and when using 5% sodium fluoride - by up to 63%. A sodium fluoride concentration of 5% corresponds to a content of 22,600 ppm fluoride ions.
Important: when choosing a toothpaste with sodium fluoride, it is important to pay attention to the fact that the paste contains only silicon abrasives (silicon dioxide, hydrated silicon dioxide, aluminum oxide, aluminum hydroxide), but does not contain calcium-based abrasives. Calcium-containing abrasives include, for example, calcium carbonate and dicalcium phosphate. Abrasives with calcium can reduce the amount of active fluoride in a tube of toothpaste - according to studies, from 15 to 50%.
A clinical study "Total and Free Fluoride Concentration in Various Brands of Toothpaste Marketed in India" published in 2015 in the Journal of Clinical and Diagnostic Research for doctors (source) found that the presence of calcium carbonate or other calcium compounds in toothpaste fluoride paste - will lead to a decrease in the concentration of active fluorine ions in the range from 15 to 50%.
Fluoride poisoning: symptoms
In sufficient quantities, the intake of fluoride into the body has a positive effect on the condition of tooth enamel. But excess intake of the substance can be very dangerous. In such situations, they talk about an overdose of fluoride - fluorosis. Dentists distinguish several forms of this pathology:
- Spotted. Small light spots form on the enamel surface and can merge with each other, forming one large spot.
- Lined. Light-colored stripes form on the teeth, which can also merge with each other. Visually resembles strokes.
- Chalky speckled. The enamel acquires a matte yellowish tint, and a large number of dots and spots form on it. This form is characterized by the appearance of dentin due to abrasion of the enamel.
- Erosive. Many specks appear. Pathology forms on the chewing surface of the teeth.
- Destructive – a dangerous form, which is characterized by increased tooth fragility.
Excessive amounts of fluoride in the body also affects the skeletal system. Among the likely complications, doctors highlight the development of osteoporosis.
Aminofluoride (Olaflur®) –
Aminofluoride (C27H60F2N2O3) is the best fluoride compound in toothpastes, but this is only its international nonproprietary name. As part of a paste or rinse, amino fluoride is usually prescribed as Olaflur®, but sometimes as Amifluor® - if the amino fluoride is combined with xylitol. Aminofluoride molecules are highly hydrophilic, due to which they attach well and remain on the enamel surface for a long time (longer than any other fluorine compounds).
Fluoride ions from aminofluoride easily penetrate even into the subsurface layers of enamel, which leads to a more significant formation of fluorapatite and fluorohydroxyapatite - compared to the use of other fluorine compounds. Also, aminofluoride tends to adsorb on dental plaque, inhibiting the metabolism of pathogenic bacteria (thereby reducing their synthesis of organic acids). In addition, it makes it difficult for microbial plaque to attach to the enamel.
Research suggests that at equal concentrations, the caries-preventive effect of amino fluoride is much more pronounced than that of sodium fluoride. According to “Prevention of dental caries using local products containing fluorides, calcium and phosphates” (authors – Popruzhenko, Klenovskaya), after application of products with amino fluoride, a much more stable layer of calcium fluoride is formed on the tooth enamel than after treatment with sodium fluoride. Those. the release of fluorine ions from CaF2 particles in this case will occur over a longer period of time.
The main advantage of amino fluoride : it begins to release active fluoride ions within 20 seconds after entering the oral cavity. This is critically important in children, because... they usually brush their teeth for only 1 to 1.5 minutes (and by the way, adults imitate them). In addition, studies (Klimek et al., 1998) have shown that the use of toothpaste with amino fluoride leads to the deposition of a more significant layer of calcium fluoride on the enamel surface compared to toothpastes with sodium fluoride.
The downside of amino fluoride is that it is difficult to produce, and therefore products containing it are expensive and, by the way, like sodium fluoride, it is also not compatible with calcium-based abrasives. It is also capable of turning the plaque layer gray, but this will only be noticeable in patients with poor oral hygiene. Aminofluoride (olaflur) – is included in Elmex toothpastes for children and adults.
Natural dental care products: components and substances
When a person thinks about his health and does not dare to buy a ready-made toothpaste with the addition of various components, he can, if desired, make a dental care product himself. This product will consist only of natural ingredients.
To make homemade toothpaste you need to take:
- 1 tbsp. l. pure white clay;
- on the tip of a knife, finely ground sea salt;
- to ensure a whitening effect, you need to add a small amount of baking soda;
- 1 tbsp. l. aloe juice or glycerin (both of these components can be added if desired, taking in equal quantities);
- 5-6 drops of lemon or mint oil.
All components are thoroughly mixed in a porcelain (glass) bowl with a wooden or glass rod. That's it, you can use it.
IT IS IMPORTANT TO KNOW! This product, prepared at home, is suitable for cleaning teeth even for children. It can be used several times during the day.
Tin fluoride –
Tin fluoride (SnF₂) – has a fairly high remineralizing ability, but is inferior in this to amino fluoride. When tin fluoride dissociates into ions - in addition to fluorine ions, tin ion (Sn2+) is also released, which has a pronounced antimicrobial effect against pathogenic microflora of the oral cavity. Currently, tin fluoride is used in toothpastes of the Blendamed PRO-Expert series, as well as in toothpastes of some other companies.
But this compound also has disadvantages, for example, tin fluoride has an irritating effect on inflamed gums. In addition, if there are foci of enamel demineralization (in the form of white spots), there is a high risk of them turning dark. And although manufacturers say that modern pastes with SnF₂ are free of these disadvantages, we are skeptical about these statements, because It is not clear why tin fluoride could suddenly stop coloring foci of demineralization.
Important: sometimes you may see that the concentration of fluoride in a toothpaste will be 1400 ppm (this is a good concentration), but the toothpaste will contain 2 different fluoride compounds at once. Typically, this may be a combination of monofluorophosphate and sodium fluoride, or sodium fluoride and amino fluoride. This is done solely for the sake of economy, and most often manufacturers in this case do not advertise the concentration of each fluoride compound in the toothpaste (indicating only the total fluoride concentration).
Water fluoridation
Saturation of water with fluoride ions is the practice of most countries in Europe and the post-Soviet space. By drinking about two liters daily, you can fully provide your body with this microelement.
But there are also opponents of fluoridation. First of all, a negative attitude towards the saturation of water with microelement ions is associated with the accumulation of fluoride in the body. They are difficult to remove and can have negative effects in the future. It is important to understand the saturation level of the water. Fluoridated water poses a real health hazard only when toxic fluorine compounds are used or the microelement concentration exceeds 1 mg/l of water.
Fluoride concentration in toothpaste –
Fluoride concentration is one of the most important criteria on which the effectiveness of fluoride toothpaste depends. The effect of fluorides is dose-dependent and, accordingly, the greater the number of fluoride ions in contact with the enamel per unit of time, the more pronounced its caries-preventive effect will be. Recommended concentrations of fluoride in toothpaste can be divided into preventive and therapeutic.
Research suggests that a fluoride concentration of 500 ppm has almost no preventive effect. Toothpastes with 1000 ppm fluoride are only slightly effective, reducing the increase in caries by only 25%. Clinical studies show that with a further increase in fluoride content for every 500 ppm, the anti-caries effect increases by 6% (compared to toothpaste containing 1000 ppm). Thus, medicinal pastes with 5000 ppm fluoride are capable of reducing the increase in caries by more than 70%.
The standard concentration of fluoride in toothpaste for the prevention of caries in adults is 1400-1450 ppm. They will reduce the risk of developing caries by 30% (and toothpaste with 1450 ppm amino fluoride will be more effective than toothpaste with 1450 ppm sodium fluoride). But in any case, pastes with such a concentration of fluoride are only suitable for patients with regular oral hygiene who rarely consume carbohydrates between main meals (snacks, cookies, sugary drinks). In patients with irregular hygiene, as well as those who consume a lot of carbohydrates between meals, this concentration will no longer be enough.
In this case, toothpastes with a fluoride concentration of 5000 ppm (Colgate Duraphat toothpaste) are recommended for daily use, or products with an even higher fluoride content, but for periodic use. The latter include the Elmex Gelee gel, which contains 12,500 ppm fluoride and is intended for use no more than 1-2 times a week. And if in Germany such drugs are allowed from the age of 6, then in Russia (according to the instructions) they can only be used in patients over 16 years of age.
Important: The amount of toothpaste you use to brush your teeth is very important. After all, the concentration of fluoride ions (F1-) that we receive in the oral fluid while brushing our teeth will depend on this. For adults and adolescents over 12 years old, this is a strip 2.0 cm long (minimum 1.5 cm). Reducing the volume of paste always reduces the effectiveness of protection against caries. However, you should always read the instructions, because... there are always exceptions. For example, the required volume of Elmex Gelee with 12,500 ppm fluoride is a strip only 1.0 cm long.
Fluoride concentrations in toothpastes for children –
Table 2 – recommended concentration of fluoride in toothpaste for children. This is data from a very authoritative organization - the European Academy of Pediatric Dentistry (EAPD). Link to scientific publication (source).
The correct amount of toothpaste for children is:
In children under 2 years of age, toothpastes containing both 500 ppm and 1000 ppm fluoride ions can be used. But in each case it is important to use a different amount of toothpaste. If you use toothpaste with 500 ppm fluoride for a child under 2 years of age, then the correct amount of it each brushing will be the size of a small pea. But if a child under 2 years old uses a paste with 1000 ppm fluoride, its correct volume will be the size of a large grain of rice. It is logical that in both of these cases, the child’s teeth will receive the same amount of fluoride ions.
Volume of toothpaste with fluoride in children under 2 years of age: (on the left – toothpaste with 1000 ppm fluoride, on the right – with 500 ppm fluoride)
What could be the consequences of microelement deficiency?
At the same time, fluorine is an important substance for our body. It affects the metabolic processes in it, promotes the removal of dangerous and heavy metals. Without it, immunity will not be supported; iron is poorly absorbed.
A lack of fluoride is reflected in the bones - they become bent, become brittle and brittle, and when fractured, they do not heal well.
Teeth also largely depend on the lack of an element: the enamel becomes thinner and becomes unusable, allowing the development of caries.
An excess of fluoride does not bring anything good either . It is not he himself, but a supernumerary amount of him. True, it can be controlled so that there is no harm. After all, too much fluoride leads to serious complications.
Best Fluoride Toothpaste – Editor's Choice
Below we will present one of the best options for fluoride toothpaste - for adults and teenagers over 12 years old. This is ELMEX toothpaste, which has an excellent caries-preventive effect in patients with regular oral hygiene. However, it must be recognized that in patients with irregular hygiene, a fluoride concentration of 1400-1450 ppm is insufficient to reliably protect teeth from caries.
ELMEX® toothpaste –
- instructions for use
- manufacturer – Colgate®,
- active substances – aminofluoride (olaflur) 1400 ppm,
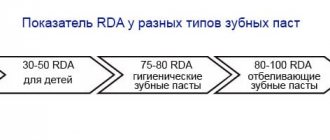
- abrasiveness – RDA 77,
- tube 75 ml – from 210 rubles.
Comments: an excellent paste for strengthening enamel and preventing caries. Contains a high concentration of amino fluoride, as well as high-quality abrasive and polishing components. It does not contain antiseptics or other additives that can only be used periodically, so it is suitable for daily long-term use. Elmex also has toothpastes for children from 0 to 6 years old - with aminofluoride concentrations of 500 and 1000 ppm, as well as for children of other ages.
COLGATE® DURAPHAT toothpaste (5000 ppm) –
- instructions for use
- manufacturer – Colgate®,
- active substances – sodium fluoride 5000 ppm,
- for patients over 16 years old,
- abrasiveness – RDA 50,
- tube 51 g – price from 430 rubles.
Comments: Colgate Duraphat 5000 ppm toothpaste is the most effective anti-caries toothpaste, which is effective even in patients with irregular oral hygiene. The paste contains a high dosage of sodium fluoride, as well as pyrophosphates (to reduce the formation of tartar). To brush your teeth, you need to use a strip of paste at least 2.0 cm long, which will ensure the required concentration of fluoride ions while brushing your teeth.
The recommended brushing time is at least 3 minutes, but we recommend that after brushing you do not immediately spit out the toothpaste foam, but rinse your mouth with it for another couple of minutes (the total time should not exceed 5 minutes). It is necessary to brush your teeth - at least 2, and optimally - 3 times a day, i.e. after every meal. The course of use is at least 1 month (at least 4 courses per year), and the rest of the time you can use Elmex toothpaste with 1400 ppm fluoride. Read about other medicinal pastes with fluoride at the link below.
→ The best pastes for protection against caries
The importance of trace elements for the body
From the above, it is quite clear that if there is an excess of fluoride, nothing good will come from brushing your teeth with a special paste. Wanting to improve their health in this way, you can achieve the opposite result - damage bone tissue and destroy enamel.
IT IS IMPORTANT TO KNOW! Fluoride has a strengthening effect on hard dental tissues, protects against caries and prevents gum disease, and minimizes bad breath.
The resulting condition is characterized by the following symptoms:
- brittle bones;
- decreased hemoglobin;
- compaction of ligaments;
- sudden weight loss;
- limitation of motor ability.
Those diagnosed with diabetes mellitus should be especially careful when using this paste, which means saturating the body with fluoride.
To prevent fluoridation from causing harm, it should be done only for medical reasons, such as, for example, the appearance of carious lesions or a general lack of this halogen in the body.
When replenishing it or using cleansing for preventive purposes, it is important to know when to stop and not to overdo it. Then the return will be effective and the benefit will be.
Excess fluoride can be harmful -
It should be noted that fluoride is not absorbed and does not penetrate the oral mucosa, and when brushing your teeth, fluoride ions penetrate only into the tooth enamel. Therefore, fluoride can only enter your body by swallowing a small amount of fluoride toothpaste or mouthwash. In children under 6 years of age, caution must be exercised here, because ingestion of paste with fluoride (provided that the fluoride content in the child’s drinking water is higher) can further increase the child’s risk of developing fluorosis.
As for the toxic effect of fluoride, this is possible when it enters the body - only in very high concentrations, which is unlikely. This is also confirmed by the missing statistics on hospitalization of children with fluoride poisoning. The probable toxic dose of fluoride is 5 mg per 1 kg of body weight. Example: in a 20 kg child, fluoride poisoning can only be achieved by simultaneously ingesting the entire 75 ml tube of toothpaste - with a fluoride content of 1000 to 1500 ppm.
How to reduce the risk of fluoride toothpaste for your child -
Fluorosis can only develop in children and only between the ages of 0 and 6 years. Moreover, a truly significant risk of developing fluorosis exists only at the age of 0 to 2 years, and in the rest of the age period the risk is regarded only as moderate. In young children, the swallowing reflex is undeveloped, and therefore small children may swallow some of the toothpaste.
According to the scientific work “The influence of inorganic fluorine on living organisms of various phylogenetic levels” (authors - Agalakova, Gusev) - swallowed toothpaste with fluoride will cause less harm under the following conditions. Fluoride absorption from the intestines is sharply reduced if the child ate calcium-rich foods, including dairy products, before brushing his teeth. This is due to the fact that calcium binds some of the ingested fluoride in the intestines, reducing it by about 30-40%.
Methods for removing fluoride from the body
Existing methods make it possible to remove excess fluoride only in the early stages of pathology development. Below we consider the main methods and means:
- Iodine helps to remove the substance in the urine, so it is recommended to consume more foods enriched with this element, for example, strawberries, some other berries, and potatoes.
- Boron - allows you to effectively remove excess fluoride from the body. Products containing this substance: honey and nuts.
- Selenium - aimed at blocking the active effect of fluoride. Brazil nuts are enriched with selenium.
- A visit to the sauna will help remove toxins through your pores.
Before you begin to remove fluoride from the body, measures should be taken to stop its entry into the body. For a certain period, patients are advised to stop eating foods rich in the element, and also stop using products containing fluoride.