Physicochemical characteristics
The substance is dehydrated stepwise when heated to 380°C to obtain glass. Dissolves in alcohols, glycerin, water, methanol. The natural mineral is borax. [5]
Sodium tetraborate – colorless crystals. An aqueous solution has an alkaline reaction. [3]
physical characteristics
- Molecular weight 381.38;
Melting point of Na2B4O7•10H2O 64°С (with decomposition);
Application
Sodium tetraborate decahydrate is used to produce boric acid and other boron compounds, as an antiseptic, and a microcomponent of fertilizers. [5] In addition, the substance is used in glass production, soldering and welding of metals, in the textile, ceramic, leather, soap, food and rubber industries, for impregnating wood and in medicine. [3] In medicine, tetraborate is used primarily in the form of a product called “borax in glycerin.” It is usually used in the fight against fungal infections of the skin and mucous membranes. When applied to damaged areas, the drug may cause irritation. [4]
Insecticide . As an insecticide, registered preparations based on sodium tetraborate are used to combat ants in food, residential, children's and medical institutions. [2]
pharmachologic effect
The action of borax is aimed at removing the cause of stomatitis and eliminating the centers of its spread. Due to the antiseptic and antibacterial effect, the drug prevents the appearance and increase in the number of microorganisms in the oral cavity.
The product is highly antifungal, so its use is justified in the treatment of stomatitis caused by fungus. Borax destroys the attachment of a fraction of the candida fungus to the mucous membrane and prevents the proliferation of its spores.
Toxicological properties and characteristics
Warm-blooded animals and humans . Sodium tetraborate penetrates the skin and has a mild cumulative effect. Does not have a local irritant effect upon contact with the conjunctiva of the eye and skin, does not cause a sensitization effect or an embryotoxic effect. [2]
People who work with the substance often suffer from chronic eczema. When working, it is necessary to protect the respiratory system, eyes and skin from exposure to dust. [3]
Sodium tetraborate, as well as boric acid and soluble borates, are quickly and almost completely absorbed from the gastrointestinal tract. In the blood, boron is evenly distributed between red blood cells and plasma, but quickly passes into the tissues. Found in soft tissues
10% of the dose (mainly in the brain, liver and adipose tissue). The excretion of boron compounds occurs mainly through the gastrointestinal tract. [3]
Hazard classes . Insecticides based on sodium tetraborate belong to class IV of low-hazard disinfestation agents according to GOST 12.1.007. [2]
Of water
In this recipe, before mixing, the water should be slightly warmed up so that the soda dissolves in it better and faster. It is more convenient to place all components into the bottle through a funnel. Then the entire measured amount will be used and not spilled or spilled.
Proven recipe
- 1 tbsp. l. water,
- 1 tsp. soda,
- 1 tbsp. l. boric acid.
Cooking method
- The specified amount of soda must be poured into a plastic bottle.
- Water is also poured there.
- Another essential component is boric acid.
- The bottle must be closed and shaken to mix both liquids and dissolve the soda in them.
- Then the composition is filtered through a cotton pad to get rid of the remaining particles of soda, and the activator can be used.
Watch this video for a DIY recipe for sodium tetraborate from water:
Story
Borax-based preparations have long been an effective remedy against cockroaches. The following recipes were used:
- take powdered sugar, flour and borax in equal proportions and in dry form, mix. Scatter in places where cockroaches often appear;
- Mix borax, flour and capsicum and also sprinkle in places where cockroaches are observed;
- mix finely ground rosin, powdered sugar and borax, sprinkle for three days where cockroaches appear;
- Dissolve borax in hot water and moisten pieces of bread with this solution. For several days in a row, place bait in places where cockroaches appear and the next day destroy the poisoned cockroaches and bait. [1]
Borax-based baits were also used against rodents - borax, powdered sugar and finely ground rosin were mixed with a plastic spatula. Sprinkle the mixture near the holes formed by rodents. [1]
Diagnosis and symptoms of stomatitis
The appearance of purulent white ulcers on the mucous membrane (stomatitis) worries children almost from birth. The causes of this pathology are not fully understood: perhaps it is a reaction of the immune system to an as yet unrecognized virus.
In children, the disease is severe:
- the mucous membrane swells and turns red;
- ulceration forms at the site of the white plaque;
- hyperthermia and general malaise are noted;
- lymph nodes enlarge;
- Sometimes ulceration appears on the surface of the lips and skin.
Stomatitis differs in the nature of the disease: acute and recurrent form. The causes of stomatitis can be caused by infection of the body or injuries to the mucous membrane, for example, a hard nipple on a bottle of formula. Stomatitis is most common in infants: the habit of trying everything on the tooth leads to inevitable injuries. In adults, mucosal ulceration can be caused by poor oral hygiene or aggressive antibiotic therapy.
A factor creating conditions for the development of the disease may be:
- acute infection;
- dermatitis;
- blood diseases;
- vitamin deficiency;
- mucosal injury;
- use of certain medications.
In most cases, stomatitis of an infectious nature occurs, caused by oral bacteria - they constantly live on the mucous membrane. A decrease in the tone of immune bodies provokes active growth and reproduction of bacteria. In children, aphthous stomatitis, which occurs against the background of immune deficiency, is more common.
Receipt
The substance is obtained by recrystallization of natural borax or kernite (tetrahydrate); interaction of boric acid with sodium carbonate; interaction of natural borates with sodium carbonate and bicarbonate when heated. [5]
Borax (sodium tetraborate) is the sodium salt of boric acid with the formula Na2B4O7. This compound has found wide application, forming variants of crystalline hydrates (crystalline type structure).
In the Asian region the substance is called "tinkal". It is believed that the ingredient got its name from the Persian word for "brown", which was used to name potassium nitrate and other fluxes containing water.
In Ancient Egypt, borax was used to preserve Mummies. Additionally, borax is used to make the glaze of pottery in China, and as a cleaning agent in several places in the Middle East and China.
The substance gained widespread use in the Middle Ages in Europe as a flux for soldering and cleaning the surfaces of metal workpieces. The nature of the origin of the substance has been a long mystery for chemists. Traces of the powder's history were eventually traced to Tibet, almost the only source that was known before the discovery (1776) and application (1820) of boric acid from Italian sources. Italy became the main source of borax until the 1860s, when the desert regions of Chile also began to supply borax for widespread use.
Read also: How to determine zero and phase without an indicator
A key figure in the discovery of the ingredient in North America was John A. Veatch, who found it in California in 1856, first in the spring at the northern end of the Sacramento Valley (Tehama County). Over the next decade, borax deposits were found in more exploitable locations in Nevada and Southern California.
American manufacturers mastered the process of using borax by the mid-1880s and supplied the product to domestic and international markets. The price fell to a level that caused the majority to refuse to use the production of such a product. Stephen Mather, to promote the substance in 1896, published a brochure: “Recipes for the Use of Borax.” This successful advertising campaign greatly expanded the demand for borax, which became relatively inexpensive, especially in laundry detergents and glass and ceramic glazes.
From boric acid powder
This recipe produces a weaker activator than traditional sodium tetraborate. It works primarily with thick office glue and is added in fairly large quantities. The slime will need to be kneaded for a long time and excess liquid squeezed out of it.
Components
- 30 g boric acid powder,
- 500 ml water.
How to cook
- Pour water into a container with a lid.
- Then boric acid powder is poured there.
- The container should be tightly closed and shaken thoroughly to dissolve the crystals.
Description of the substance
What is borax substance? Many minerals containing sodium tetraborate have been studied. Such deposits include:
- borax or sodium tetraborate tinkal decahydrate;
- kerite;
- Borax sediment is formed during the drying up of salt lakes (Searles, lakes of Turkey);
- minerals belonging to the borate class, containing calcium, sodium and similar elements other than borax.
Varieties of borax
There are two options for this material. In solid form, borax appears as a powder with small granules of a small fraction with a white color. The flux will not spread during application and can be precisely positioned in the required metal-to-metal junction areas.
Diluted borax has also been used for light metals. In some cases, liquids are very convenient, because you just need to dip a small part into the solution. In addition, the liquid form allows the use of borax even at low heating temperatures.
In some cases, it is advantageous to use a mixture of fluxes with borax included in the composition for the special characteristics of the base metal and special requirements for the connection.
Slime with tetraborate from PVA glue
In this recipe, artificial snow does not need to be previously combined with water for swelling; it is added to the slime in its pure form. Daiso clay can be replaced with light plasticine. But if you don’t use either one or the other, the slime will still turn out unusual and interesting.
Ingredients
- 60 ml PVA glue,
- 2 doses of liquid soap,
- 2-3 drops of liquid paint,
- 1-2 tsp. artificial snow,
- 0.5 tsp. diluted sodium tetraborate,
- 1 small piece of Daiso clay.
How to make
- The PVA must be transferred to a mixing cup.
- Liquid soap should be added to it.
- The components are immediately mixed.
- To give the mass a bright color, dye is added.
- It needs to be shaken until the shade is even.
- Next, artificial snow is poured into the liquid, stirred, and the mixture is allowed to stand for several minutes.
- Then an aqueous solution of sodium tetraborate is poured into the base in portions.
- The mixture must be stirred with a spoon until it stabilizes.
- It is kneaded a little in your hands.
- All that remains is to mix the slime with Daiso clay.
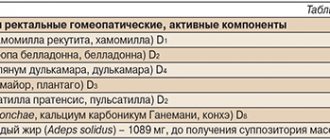
Composition and physicochemical properties
| Preparative form |
Borax is a compound of soda and boric acid. This substance does not dissolve in alcohols, but forms solutions well with hot water and glycerin.
Molecular structure of sodium tetraborate
Borax reacts with strong acids to form a salt and boric acid. When heated above 400 °C, the substance is completely deprived of water in the crystals. Borax, as a salt of a weak acid, when mixed with water, creates an alkaline reaction with sodium tetraborate. Borax can react with certain metal oxides to form various compounds - borax pearls.
As a food preservative, borax is prohibited for use in most countries, including Russia, due to the inability of the substance to be removed from human organs as a toxic ingredient. As a food additive, the material is called E-285.
Chemical reactions involving borax
Sodium tetraborate for slime
The substance sodium tetraborate is found in the children's entertainment toy slime. Knowing how to make sodium tetraborate at home, you can make a toy for your child from the substance. It will be no different from the store-bought version, but since it is a chemical, it is advisable to play with it carefully.
Homemade slime for your child will have more significant advantages compared to purchased options. To make a toy yourself, you first need to make a borax solution, and also prepare glue (preferably transparent), water and gouache, you can use food coloring.
Slime making process:
- find a container and pour warm water into it;
- add the prepared borax solution and mix the composition well;
- after dissolving the substance, we find another container and pour half a glass of water, glue and dye into it, and then also mix as much as possible until a homogeneous mass of the same color is obtained;
- now the mixture is poured in a thin stream into the container with the dye, but at the same time you need to mix the composition;
Ready-made slime
The result is a viscous mass that a child can play with, but you need to control it so that the baby does not put the slime in his mouth.
We discussed other options for making a child-safe slime toy in a separate article.
We also recommend reading the material on how to make chewing gum for your hands.
Scope of application of the drill
Sodium tetraborate has been actively used for the following purposes:
- as a flux during soldering and melting of metals;
- in analytical chemistry studies as a standard substance for determining the level of acid in a solution. Borax is also used to establish the characteristics of metal oxides;
- widespread use in the creation of glazes, enamel, glasses for optical instruments and decoration;
- the powder is used in pharmaceuticals and paper production;
- is a natural preservative and means for disinfection and control of parasites;
- is a component in the chemical industry to create household cleaning products;
- relevant application in the creation of cosmetic products;
- borax is used as a base for creating boron;
- the substance is a component for creating insulating building materials;
- in light industry, sodium tetraborate is applied to the product before the painting procedure.
Using a drill in everyday life
Borax can be found in most grocery stores. It is a relatively inexpensive product, making it an excellent choice for many household projects.
Use of sodium tetraborate as a medicine
The substance is very effective in controlling pests: cockroaches, ants and other household insects. The mixture is ready by mixing equal parts of powder with sugar. Sugar helps attract beetles and borax exerts its detrimental effect on the insect. It is recommended to keep the substance in hard-to-reach places, away from pets and children. Optimal places: under stoves, refrigerator and sink. Borax also works well against mice. You just have to apply the powder in the areas where the mice are located, and the borax will rid you of the pests. You can also sprinkle the solution on the carpet and vacuum it to eliminate the presence of fleas or treat the mattress to get rid of bedbugs.
Read also: RGB tape what is it
Borax will get rid of rust. Mixing 1 cup of powder with 2 cups of water and 1 tablespoon of lemon juice makes an effective anti-corrosion agent. The paste-like solution is applied to the rusty items for about 15 minutes. After which the rust can be easily removed by mechanical friction.
Sodium tetraborate is a universal cleaner. Two tablespoons of borax are mixed with 2 cups of water to create an all-purpose cleaner. The solution can be applied in a spray bottle and used to clean kitchen surfaces and bathroom tiles and ceramics. Borax is great for removing very stubborn stains. Borax will help remove stubborn stains from the floor.
Borax will allow you to flush your home plumbing fixtures. Simply place ½ cup of borax into the drain with a few cups of warm water. Borax breaks down dirt that gets stuck in pipes. This will not only remove excess and unclog the drain, but also disinfect the system.
Application in forging and forge welding
Borax is actively used as a flux for forging and forge welding. The powder is a source of boron oxide, with excellent antioxidant properties. Borax can, if necessary, remove small cracks during metal processing, change the shape of a product, or when heated during artistic forging and blacksmithing of metal. The substance is classified as a high temperature flux. The processed workpiece with borax is characterized by more wear-resistant characteristics and durability.
Using borax in soldering
During the melting of borax at a temperature of 700-900 °C, the surface of the material being processed is cleaned, and all excess inclusions are dissolved in the flux. During the processing of a material by forging, a thick layer of scale is gradually created. In some cases, the metal being processed may completely burn due to overheating of the part. But by using a thin layer of borax, this scenario can be avoided.
Slime with tetraborate analogue - washing gel
The recipe uses concentrated Persil gel, but Perwoll or Laska are suitable for the same purpose. You will need a lot of activator. Still, it should be poured in little by little, and the base should be thoroughly stirred, first with a spatula and then with your hands.
What do you need
- 100 ml PVA glue,
- 1 pinch of powder dye,
- 6 tbsp. l. shaving foam,
- 2-3 tbsp. l. Persil liquid washing gel.
Cooking method
- The glue is placed in a bowl and dye is immediately added to it.
- You need to mix everything so that the color of the future slime appears.
- Next, shaving foam is squeezed into the composition.
- The base must be mixed again.
- Then Persil gel is poured in parts.
- Stir the mixture with a spoon until it thickens.
- Then it is kneaded manually until it comes away from the skin.
How is the soldering process performed?
Brazing is a process in which two or more metal elements are joined by melting, using a filler metal in the joint that has a relatively low melting point. Soldering is used to form a permanent connection between components. During soldering, only the solder melts, and not the parts of the material that were soldered. Solder is a metallic “glue” that holds parts of materials together.
Soldering copper pipes
If you need to create a flux from borax, you can use boric acid in a 1:1 ratio to work with copper, steel, cast iron or a similar substance. This mixture must be mixed and then the excess liquid must be evaporated to create a dry type of flux. This preparation makes it possible to obtain an active, high-quality flux for processing parts.
Borax soldering is one of the most common solder processing options. The method allows you to successfully install or adjust parts and various mechanisms.
Advantages of using technical drill for soldering:
- such soldering can connect different metals into one element;
- materials can be used with different melting temperatures;
- soldering allows metals to be bonded to non-metallic materials;
- connections created by this method can be destroyed by repeated heating if necessary;
- A special feature of soldering is the absence of melting of the base metal during operation. In this way, the master can avoid warping, changing geometric shapes and other changes;
- borax creates good conditions for soldering parts and setting them;
- powder allows you to achieve high strength.
Causes of errors and their correction
There are several reasons that prevent you from making slime:
- Wrong choice of glue . It is better to opt for PVA glue from. You can also use silicate glue from various manufacturers. These types of glue are sold in bookstores and office supply stores. When purchasing, you need to pay attention to the production date.
- Expired sodium tetraborate . An expired product is less active.
- Not maintaining proportions . Add tetraborate drop by drop and mix thoroughly.
You can fix a failed slime in the following ways:
- If, when preparing the mixture, glue was used that has lost its properties, then a film mask is used to correct this. It is added to the mass. If it does not begin to thicken, you can add more borax.
- If the glue does not thicken even after adding tetraborate, you can add washing gel, starch or lens care liquid instead.
- Sometimes it happens that drills add more than the required amount. The glue begins to curl into flakes or the slime becomes very hard. In this case, do the following:
- add more glue;
- use a film mask;
- add cream or gel to the mixture;
- heat or add warm water.
Features of the soldering process
There are various soldering options. Many of them have a similar operating algorithm.
Submerged Soldering
For a quality connection, the following steps are necessary:
- The solder must be applied to a clean surface that acts as a base. It is necessary to remove any oils, paints, waxes and other impurities using a solvent, steel brush, or fine sandpaper.
- In order for the solder to connect to the tip of the soldering iron, heat it up for a few seconds, and only then apply the solder. Hold the soldering iron like a handle, near the base of the tool.
- Both parts of the workpiece that will be soldered must be hot to form a good bond.
- The tip of the soldering iron heats both sides of the workpieces.
- The solder will not flow well on the heated base metal. Enough solder should be used to form a strong connection.
- Remove the tip from the joint area as soon as the solder begins to flow.
- Do not move the solder joint while the solder is cooling. Do not overheat the connection as this may cause damage. Transistors and some other components may be damaged due to the heat of soldering. The alligator clip can be used as a heat sink to protect these components. By absorbing heat, the alligator clip reduces heat, helping prevent damage.
- Soldering a connection can only take a few seconds, and even an amateur can perform the operation. If the seam looks bad, reheat it and try again. Bad bonds (also called dry bonds) must be melted and remade. Wipe the tip of the soldering iron to clean it. Unplug the soldering iron when not in use.
Read also: What is the difference between zero and zero
Video
You can avoid mistakes when making slime if you follow all the instructions from the video. The main thing is to observe the ratio of ingredients and mix them in the specified order.
You can learn more about how to make slime by watching the video below:
Any slime requires proper handling . They should be stored in a special container. It is left in the refrigerator. Slime easily loses its elasticity when exposed to heat. If a toy becomes dirty, wash it with water. This will allow you to keep the gum for your hands for a longer time.
How to solder copper pipes
Before starting the operation, prepare all the required tools and materials:
- various brushes for cleaning the edges of pipes for soldering;
- tools for monitoring heated workpieces before soldering;
- equipment for thermal heating of workpieces. Most often a gas burner is used;
- Solder, drills as flux and brushes for applying the material are also required.
For the soldering operation with borax, not including the flux preparation step, it can be divided into various stages:
- The master cleans the workpieces from excess dirt and inclusions. Using fine sandpaper, the area where the pipe pieces are joined should be cleaned to a characteristic shine.
- After cleaning with a brush, you can apply borax. The substance must be applied to the outer and inner sides of the joint. Products with a layer of flux are connected at the joint to begin soldering.
- The pipe mass is heated with a gas heat source for at least 20 seconds.
Solder is applied to the contact area and also processed with a melting torch. The solder is distributed evenly throughout the joint area. If the seam passes visual inspection, it can be tested under working conditions.
Authenticity
1. Qualitative reaction. To 5 ml of a 4% solution add 0.1 ml of a 0.1% phenolphthalein solution; the solution turns red. When adding 5 ml of glycerin, the 85% solution should become colorless.
- Qualitative reaction. To 0.2 g of the substance add 1 ml of concentrated sulfuric acid, 3 ml of 96% alcohol and mix. When ignited, the mixture should burn with a green-edged flame.
- Qualitative reaction. A 4% solution should give a characteristic reaction A to sodium (General Pharmacopoeia Monograph “General reactions to authenticity”).