Margarita Morozova “Popular Mechanics” No. 3, 2018
The shock and awe of visiting the dentist is familiar to us since childhood. And many adults feel overwhelmed by the jingling of instruments, and sometimes by the mere sight of a dental clinic. As a result, despite all the successes of modern medicine, there is practically no hope for a future without caries. But it is this, together with periodontitis, that is the main cause of tooth loss in people of all ages. The problem stimulates the search for new treatment methods, including in the field of bioengineering. Methods that will allow you to forget about fillings and crowns and simply grow new healthy teeth instead of damaged ones.
Tissue engineering in dentistry has been used since the era of the pharaohs: the oldest known dental implants were found by archaeologists in Egypt. Among them are teeth that were reimplanted into the woman in place of the lost ones and were partially integrated with living tissue. An artificial tooth was discovered in a man's jaw, expertly carved from a mollusk shell 5,500 years ago. But despite the impressive period, complete treatment for a patient with adentia, that is, complete or partial loss of teeth, still does not exist.
Background
around 330 BC Aristotle describes the regeneration of the tip of a lizard's tail. 1950s Two types of stem cells are found in the bone marrow: hematopoietic precursors of blood cells and mesenchymal precursors of bone and cartilage tissue, including teeth. 1981 For the first time, mouse embryonic stem cells have been isolated and grown in vitro. 1985 Stem cells have been found in human dental pulp. 1998 Successful isolation and laboratory cultivation of human embryonic stem cells. 2000 It has been shown that mesenchymal stem cells in the pulp are capable of regenerating dentin-like tissue complexes. 2003 It is possible to isolate a population of stem cells from the still living remains of a decayed tooth. 2004 Finding stem cells in the periodontal ligament, which holds the tooth in place. 2006 Mature differentiated cells from mice have been successfully “reprogrammed” into stem (induced pluripotent) cells. 2007 Induced pluripotent stem cells are derived from human fibroblasts. 2010 Dentin-like complexes were grown from pulp stem cells on an artificial scaffold.
Own or artificial
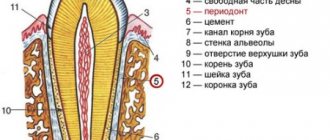
Orthopedic structures and implants to some extent compensate for the functions of a lost tooth, but these artificial substitutes lack blood vessels, nerve endings and receptors. In addition, they do not form the periodontal ligament, the layer of connective tissue between the tooth root and the bone that forms the wall of the socket. The periodontium helps secure the tooth in the alveolus and ensures its mechanical stability: the strength of the human chewing muscles is as much as 390 kg, and the ligament distributes this pressure between the teeth.
Unlike a tooth, an implant is immobile, and the development of connective tissue around it often ends in inflammation (peri-implantitis) and requires removal of the artificial tooth. In addition, the implant cannot be connected into one structure with the patient’s teeth precisely because of the inability to adequately distribute pressure due to the absence of periodontal tissue. Finally, an implanted substitute requires a much more careful attitude to oral hygiene, which again brings us back to the main source of our problems, the “human factor”. Obviously, the ideal solution would be the technology of growing real living teeth, rather than transplanting artificial ones. So let's get down to business.
The earliest sign of tooth development is the formation of the dental lamina, a horseshoe-shaped thickening of epithelium that extends along the upper and lower jaws of the embryo. After passing through several stages, it forms the roots of individual teeth. Coordination of this process is ensured by at least four epithelial signaling centers, the cells of which secrete substances that regulate tooth formation.
All of the above will also be useful to us for creating new teeth using tissue engineering methods. The “recipe” for growing any biological tissue requires three basic components: stem cells, an extracellular matrix (the scaffold that provides support for developing cellular structures) and, finally, growth factors, combined into the signaling pathways necessary for tooth development. Let's go in order and start with the main characters - stem cells that have odontogenic competence and are capable of developing into dental tissue.
Bolotov method
Strictly speaking, Bolotov’s method does not concern the regeneration of teeth, but their treatment, but as an alternative it makes sense to consider it in more detail. The regeneration process is based on a combination of vodka tinctures, propolis and calamus. For 0.5 vodka, take one calamus root and crush it, propolis tincture is made at the rate of 20 grams per half liter.
The technique is based on the simultaneous use of both tinctures and involves the following course of action: one tablespoon of each tincture is mixed together and the resulting mixture must be rinsed out of the mouth for three minutes. It is not recommended to swallow this composition; rinsing should be done either at the onset of pain or before bedtime. The duration of treatment is approximately a month, although pain should subside 2-3 after the start of the procedure. Calamus acts as a pain reliever, and propolis is necessary for filling holes in teeth.
Note: This procedure cannot be called regeneration; new teeth cannot be grown with its help, but pain completely disappears and even roots that do not sit well in the gums become stronger.
Dental stem cells
Unlike most mature cells, stem cells are able to undergo many divisions and gradually specialize, forming different types of cells. Embryonic stem cells are totipotent and can develop into any of more than 200 types of adult cells. Postnatal stem cells remain in the tissues of the adult body. They are multipotent, that is, they are able to give rise to only certain types of cells, and are localized in the corresponding tissues, be it bone marrow, blood vessels, liver, skin or dental tissues.
Depending on the location, dental stem cells (DSCs) are divided into stem cells of the pulp, extracted primary teeth, periodontal ligament, gums, dental follicle precursor cells, etc. This gives us many opportunities to obtain them. Pulp stem cells can be isolated directly from extracted teeth - this is a convenient and promising source of DSCs, suitable for the restoration of both dentin, pulp and cement, and bone tissue. In addition, they exhibit pronounced neuroregenerative activity, inhibiting the death of neurons, astrocytes and oligodendrocytes after injury, accelerating the recovery of damaged axons. The population of stem cells from extracted primary teeth can differentiate into cells of bone and nerve tissue, and DSCs from the gums are suitable for the restoration of periodontal tissue, muscles, and even tendons.
The mechanisms of development of odontogenic stem cells have not been fully elucidated, but more than 200 genes operating in them have already been identified. It is clear that each type of DSC has its own characteristics, which promise their use not only in dentistry, but also in other areas of medicine. Another stem cell resource for growing teeth remains induced pluripotent stem cells (iPSCs), obtained by “reprogramming” adult differentiated cells through treatment with a special cocktail of signaling molecules. Scientists continue to develop safe methods for creating iPSCs and using them.
Intercellular matrix
But the resource of stem cells for growing teeth is not even half the battle. For the development and formation of a complex structure of mature tissue, a support, a scaffold of intercellular matrix molecules is required: it is this that supports the attachment, migration and spatial organization of cells. The gaps and pores in it ensure the movement of cells, growth factors and metabolism. An artificial scaffold must be easy to use, biocompatible, degradable in the body and low immunogenicity, good mechanical properties, etc.
Among the synthetic materials for scaffold formation, it is worth mentioning “bioactive” glass, which can grow together with biological tissues, polylactic acid and composites based on metal, ceramics or polymers. All of them make it possible to produce scaffolds of the required shape, although their use remains very limited due to low biocompatibility and toxicity. In contrast, natural biomaterials for scaffolds—such as collagen, chitosan, or hyaluronic acid—are biocompatible and easily biodegradable. However, they are less durable and can cause rejection reactions.
In any case, the ideal scaffold material would be a structure derived directly from natural extracellular matrix polymers or from their synthetic analogues. Pulp and periodontal stem cells growing on such a scaffold, when treated with appropriate signal substances, successfully developed in the odontogenic direction - towards the formation of tooth tissue. However, we will return to this later, but for now we need a third type of ingredients.
Signaling pathways
Stem cells are our main resource, the scaffold is the basis for its development, but their interaction must be orchestrated by signaling molecules, including growth factors and interfering RNA. Growth factors are peptide molecules that transmit signals to control cellular behavior through action on specific receptors on the surface of cells. They provide interconnection and interaction between cells, as well as between them and the extracellular matrix. So, if the carious cavity is close to the sensitive pulp or the patient experiences increased tooth wear, the corresponding growth factors trigger the formation of secondary and tertiary dentin. A number of growth factors have also been identified that act during tooth development, such as bone morphogenetic protein (BMP), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF). They are delivered to stem cells using nanoparticles or through the scaffold itself, filling it with the desired set of molecules.
Interfering RNA molecules are also used to control cell differentiation. They bind to messenger RNA and stop the synthesis of a particular protein. For targeted delivery, such RNA is converted into DNA and transferred into a cell in the form of a plasmid. Now we have everything we need to obtain a tooth: dental stem cells (assorted), scaffold (product identical to natural) and growth factors (to taste).
Blame evolution
Human teeth have been destroyed by progress. If we take the Ardipithecus, which lived 5.8–4.4 million years ago as our starting point, and probably gave rise to the Australopithecines (and humans already descended from them), it turns out that our ancestors were omnivores. Of the living primates, Ardipithecus was most similar to chimpanzees. Most likely, they also used tools: they used sticks to extract insects from termite mounds and, more importantly from a dental point of view, they cracked nuts with stones instead of gnawing them.
Omnivory and tools had already made the teeth of human predecessors less wear-resistant than those of purely herbivorous primates like orangutans (but we must understand that no one descended from orangutans). These are the costs of versatility: an unspecialized instrument can do a lot, but it’s unlikely to do any of it masterfully. An “omnivorous” tooth will not have super-thick enamel or incredibly sharp cutting surfaces, but it can somehow grind almost any food.
Skull of Ardipithecus ramidus
). The age of the find is 4.4 million years.
Irina Efremova / wikimedia commons / CC BY-SA 4.0
Share
Subsequent improvements in the quality of life - thermal processing of food (in other words, the use of fire), cutlery and an abundance of ready-made meals - further weakened human teeth, and in addition ruined the bite. It sounds Lamarckian, but it seems to be true: “lack of exercise” of teeth has led to the fact that in people they have become of little use.
The food became softer and the jaws became shorter. But the number of teeth did not want to decrease, and even now it does not want to. Nowadays, it’s rare for anyone to have all their teeth straight into their given places at once: more and more often they have to be straightened with braces and other similar instruments, and the outermost “wisdom” teeth have to be removed.
Agriculture and the war against rodents played a cruel joke. About ten thousand years ago, people learned to grow the necessary plants and began to domesticate animals - all for use as food. Having gained some control over their own diet, Homo sapiens preferred the caloric content of grain carbohydrates and the protein of domestic animals to the uncertainty of collecting wild fruits and free, but too lean game. This was due to the bacteria - the inhabitant of the surface of the teeth, the culprit of caries - Streptococcus mutans
.
One could blame bread and sweets for everything, but it turned out that the harmful microbe only took advantage of the opportunity and cleverly adapted to the changed diet of the new owner. Streptococcus mutans
- the same age as agriculture, and there is a high probability that we got its ancestors from rats, but not from monkeys or hamsters.
At least,
our streptococcus is the closest relative of Streptococcus ratti How the bacterium jumped from rat teeth to ours is a separate question.
The recipe is ready
The basic principles of dental tissue engineering have already been developed, and attempts to move on to practical application have been made for more than a decade and a half. English scientists can be called pioneers in growing teeth, who began such research back in 2002. And although their experiments on the regeneration of hard dental tissues did not bring much results, soon scientists from Takashi Tsui’s team conducted more successful experiments that lasted about two years. After solving a number of problems, they managed to isolate dental stem cells from mouse embryos, “assemble” a bioengineered rudiment from them, grow a full-fledged tooth from it and implant it into the jaw of a mouse.
The protocol prepared by Japanese specialists during this work has become one of the key guidelines used by scientists for experiments in the field of tissue engineering. Russian scientists from the Evdokimov Dental University (MGMSU) also relied on it: in 2021, they managed to conduct their own successful experiments in growing mouse teeth. Human teeth are more complex and bulky, and it is not yet possible to grow them. Problems remain unresolved related to the innervation and blood supply of the “bioengineered” tooth, its ligamentous apparatus, and most importantly, the choice of the pool of stem cells.
The fact is that you can get human DSCs from a healthy tooth (by damaging it) or from a tooth with the pulp removed. Available cells, such as gum stem cells, do not have odontogenic ability. It is still yet to be learned how to obtain the necessary DSCs from existing resources or induced pluripotent stem cells. However, there is no doubt that after some time, bioengineering of teeth will help both adults and children completely forget about the trepidation before visiting the dentist.
Technology of influencing the subconscious at home
Supporters of spiritual practices believe that the power of thought will help grow new teeth. The active work of consciousness will “awaken” the regeneration mechanism. You need to clearly convey your intention to the body, and have no doubt that self-healing is possible. Then a positive result will be achieved.
Mikhail Stolbov, author of the book “How I Grew New Teeth,” described practical exercises. He recommends the following:
- visualize or remember the sensations that accompanied the emergence of young teeth in childhood - itching in the gums, pushing out baby teeth by emerging molars,
- it is advisable to start restoration from the lower incisors in the same sequence as they emerge in infants,
- the subconscious must “work” towards tooth regeneration for 24 hours,
- It is important to familiarize yourself with the technique of growing new teeth and watch thematic videos several times.