Chlorhexidine belongs to the group of broad-spectrum antiseptic and bactericidal drugs for external and local use. It is based on the substance bigluconate, as well as purified water or alcohol. The solution is available on the market in different concentrations. In this article we look in more detail at what chlorhexidine solution is and where it is used.
Application area
The main area of application of chlorhexidine bigluconate is medicine. In various forms it is used to treat runny nose, pharyngitis, laryngitis, stomatitis. This is an effective drug for the prevention of sexually transmitted diseases.
Chlorhexidine can be used both for the treatment and prevention of numerous diseases. But here it is important to choose the right concentration. For example, solutions containing a substance of 0.05─0.5% are used for applications and rinses. Gels with a concentration of 0.5% are used for external and local use. To rinse the mouth, use an aqueous solution with a concentration of 0.2% or an alcohol solution with a concentration of 0.1%.
The use of the drug for hand disinfection is popular; it is an excellent antiseptic: at the height of the coronavirus epidemic, its use has increased significantly.
The substance is used in surgery to treat the hands of medical personnel; it is also used to sterilize instruments; for this purpose, a half-percent alcohol solution or one-percent aqueous solution is used. The injection sites are treated with the product.
Due to its effective disinfecting effect, the places where bigluconate-based antiseptics are used are:
- Children's institutions: kindergartens, schools, colleges, etc.
- Communal facilities: baths, swimming pools, public toilets, etc.
- Sports facilities: gyms, fitness centers, etc.
- Beauty salons: hairdressers, beauty salons.
- Places of recreation and entertainment: nightclubs, cinemas, museums, theaters.
- Catering: canteens, bars, restaurants.
In addition, antiseptics are actively used in resorts, sanatoriums, cultural and health complexes, social security institutions and in everyday life.
Infections caused by antibiotic-resistant microflora represent an ever-growing threat in both hospital and community settings. Nosocomial infections lead to a decrease in the effectiveness of therapy, an increase in the duration of hospitalization and an increase in mortality.
Chlorhexidine bigluconate was developed in Great Britain in 1950. It is the first internationally recognized antiseptic for skin and wounds. One of the advantages of chlorhexidine, in addition to its pronounced antimicrobial effect, is its ability to bind to various biological substrates while maintaining its antibacterial activity, and then be slowly released, which leads to the preservation of effective concentrations of the drug. To date, there are no reports of resistance to chlorhexidine, despite more than 60 years of active use of the drug in the clinic. Chlorhexidine gluconate remains important in the prevention of nosocomial infections.
Widespread use of antiseptic methods for the prevention and treatment of infections followed the publication of Joseph Lister's The Antiseptic Principle in Surgical Practice in 1867. At the same time, “Lister’s antiseptics” met ardent opponents, whose main argument was the toxicity of the antiseptic they used (carbolic acid). Despite the availability of numerous antiseptics, the question of the safety and effectiveness of this group of drugs remains constantly relevant. The “longevity” of chlorhexidine and the prospects for its further use as one of the most powerful antiseptics widely used in clinical practice is a pressing issue for clinicians.
A cationic detergent (detergent), later named chlorhexidine, was synthesized during the development of antimalarial drugs in 1947. This compound has bactericidal antimicrobial activity, especially against gram-positive microorganisms. Of the 10,040 compounds, the first to enter the market was chlorhexidine gluconate, registered in 1954 by Imperial Chemical Industries Co.LTD (UK) as “Gibitan” - the first internationally recognized antiseptic for the treatment of wound surfaces and skin. In 1957, only 3 years after entering the market, the indications for its use were expanded to include not only skin treatment, but also use in ophthalmology, urology, gynecology and otorhinolaryngology. In 1959, chlorhexidine began to be used to control bacterial plaque, leading to its widespread use in dentistry. Currently, in clinical practice, chlorhexidine is preferred not only when treating the skin (hands, surgical field), but also as an oral antiseptic, including for the prevention of nosocomial infection. The most commonly used concentrations are 0.2% and 0.12% solutions. In addition to its effect on plaque and gum condition (gingivitis), chlorhexidine is effective in the prevention and treatment of caries, secondary infections after dental procedures or implant placement. Chlorhexidine reduces the bacterial load and the risk of bacteremia after dental procedures. It is also used in the treatment of recurrent atrophic stomatitis and stomatitis associated with the installation of dentures, primarily in groups of patients with orthodontic appliances and immune disorders. One of the main advantages of chlorhexidine, in addition to its powerful antimicrobial effect, is its ability to retain its antimicrobial activity when bound to various substrates. At the same time, it is released slowly while maintaining an effective concentration. This property is known as substantiveness. Chlorhexidine is not susceptible to blood, pus, or saliva. Chlorhexidine is pharmaceutically incompatible with soap and detergents (for example, those containing lauryl sulfate), alkalis and other anionic compounds (colloids, gum arabic, carboxymethylcellulose), and iodine. Compatible with ethyl alcohol, benzalkonium chloride (contained in contraceptives for local use Pharmatex and Benatex). Ethanol enhances the effectiveness of the drug. The bactericidal effect increases with increasing temperature. At temperatures above 100°C, the drug partially decomposes. Used in a neutral environment; at pH 5-8 the difference in activity is small; at pH above 8 it precipitates. The use of hard water reduces bactericidal properties. Compatible with drugs containing a cationic group (benzalkonium chloride, cetrimonium bromide).
The unique combination of properties of chlorhexidine also determines the variety of dosage forms.
Chlorhexidine is available in the following forms: • 20% concentrated solution – intended for dilution before use, used in medical institutions; • 0.05% solution in plastic and glass bottles of 70 and 100 ml - used without dilution, including at home; • Vaginal suppositories (suppositories) “Heksikon” containing 0.016 g of chlorhexidine, 1 or 10 suppositories in a package; • Vaginal suppositories (suppositories) “Heksikon D” containing 0.008 g of chlorhexidine – intended for children, 10 suppositories per package; • Gel containing 0.5% chlorhexidine. Chlorhexidine is included in the following preparations: • Solutions for mouth rinsing for dental diseases and manipulations (tooth extraction, opening of abscesses, professional cleaning, etc.) – Elgidium, Amident, Eludril; • Gels for gums, intended for the treatment of dental diseases and pain relief in the oral cavity (for example, when getting used to dentures), one of the components of which is chlorhexidine - Dicloran Denta, Elugel, Metrohex, Parodium, Elgifluor, Dentamet, Metrogyl Denta; • Elgidium toothpaste; • Solutions with other antiseptics – Baktoderm (with benzalkonium chloride), Chlorhexidine alcohol spray – for the treatment of skin infections, Citeal (with hexamidine and chlorocresol) – for topical use in gynecology, dermatology; • Ointments Bepanten plus and Depantol (with panthenol - wound healing effect), Bemilon (with betamethasone - anti-inflammatory effect); • Vaginal suppositories Depantol (with panthenol) – used in gynecology after surgical interventions; • Chlorhexidine in combination with lidocaine for local anesthetic action in the Lidocaine-Asept spray and Instillagel and Kategel gels with lidocaine; • Lozenges for sore throat, stomatitis and other diseases of the oropharynx and oral cavity – Hexoral tabs, Anti-Angin formula, Sebidin.
Chemical properties
To better understand what chlorhexidine is, you need to know its chemical properties. It appears to be a white crystalline powder and has a melting point of 137 °C. To make the drug, the powder is diluted in a certain proportion with water or alcohol. The resulting solution is colorless or light yellow.
It has a weak, unexpressed odor or is characterized by its complete absence. The chemical formula is C22H30CL2N10. It is stable, which makes it possible to achieve a normal bactericidal effect when applied to the skin, when rinsing, etc. When mixed with alcohol, the bactericidal and antiviral characteristics of the substance are enhanced.
Chlorhexidine and coronavirus
It has been proven that alcohol-based antiseptics are effective against coronavirus, and its content should be at least 60%, but preferably more. Therefore, chlorhexidine in an aqueous solution effectively copes with bacteria, but not with viruses.
But, as we said above, chlorhexidine is also made with alcohol, which makes this antiseptic effective in the fight against COVID 19. To do this, you can purchase an alcohol solution of chlorhexidine with a concentration of 0.5% at the pharmacy. Another option: take a 20% aqueous solution of the substance and a 70% alcohol-containing liquid and mix these two components in a ratio of 1 to 40. You need to treat your hands with the composition according to this principle:
- First, wash your hands with soap for 15 seconds.
- Then rub in the alcohol solution of chlorhexidine for 2 minutes.
- The amount of liquid rubbed in should be approximately 5 ml.
But you can wipe surfaces with aqueous solutions; with prolonged contact with the pathogen, most of the virus will still be destroyed.
Often, antiseptics with chlorhexidine are produced in the form of a gel containing alcohol and depanthenol, which moisturizes the skin.
Medical Internet conferences
Chlorhexidine is an antiseptic that does not lead to bacterioresistance
Pogosyan M.A.
Scientific supervisor: assistant of the Department of Pediatric Dentistry and Orthodontics, Ph.D., Kazakova L.N.
GBOU VPO Saratov State Medical University named after. IN AND. Razumovsky Ministry of Health of the Russian Federation
Department of Pediatric Dentistry and Orthodontics
An analysis of Russian and foreign medical literature has shown that for more than 60 years of active use of chlorhexidine in the clinic, there are no reports of microflora resistance to it.
Chlorhexidine is an amphipathic molecule with hydrophilic and hydrophobic groups and is a cation at physiological pH. The molecule consists of two symmetrical chlorophenol rings (4-chlorophenyl) and two biguanide groups united in the center by a hydrophobic hexamethylene chain, being a symmetrical molecule - 1,6-bi-4-chlorophenyldiguanidohexane [1, 2]. Typically, chlorhexidine is used in the form of salts, mainly biacetate, bigluconate or dihydrochloride [3,4,5]. Bigluconate is most soluble in water and alcohols [5]. In addition, this form has the additional advantage that the active components with a positive ionic charge are released at physiological pH values. Chlorhexidine is practically not absorbed from the gastrointestinal tract. After accidental ingestion, the drug is not detectable in the blood after 12 hours. Due to its cationic nature, chlorhexidine binds well to the skin and mucous membranes. Chlorhexidine is not inactivated by blood and plasma proteins, is distributed locally when applied cutaneously and is practically not involved in metabolism. Chlorhexidine is widely used in medicine as an antiseptic and disinfectant for surfaces and instruments. For diseases in surgery, dentistry, gynecology, urology, dermatovenereology, otolaryngology, it is used locally. It is the main antiseptic for treating the surgical field, surgeon's hands, and medical staff's hands in postoperative care for patients in ENT departments and in cosmetology. Chlorhexidine is characterized by a pronounced antibacterial effect and substance compared to other antiseptics used to treat the oral cavity. [6]. At a dental appointment, chlorhexidine is offered in concentrations of 0.05%; 2% in the form of solutions and Diplen-Denta X, containing chlorhexidine digluconate 0.01-0.03 mg per 1 sq. cm.
What concentration of chlorhexidine has the best properties, which is more effective in the treatment of caries of primary and permanent teeth in children?
Purpose of the work: to compare the effectiveness of chlorhexidine bigluconate at a concentration of 0.05%; 2% in relation to microflora taken from the carious cavity at different depths.
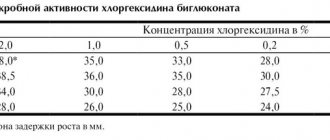
Materials and methods : we conducted a study of the quality of the microflora of 20 carious cavities: 10 in baby teeth and 10 in permanent teeth. Infected dentin of the carious cavity was taken with a sterile bur, bacteriological examination was carried out according to all the rules of clinical microbiology, to isolate aerobic and optionally anaerobic microorganisms. During primary culture, multiple streptococci and staphylococci were isolated from carious cavities and in milk and permanent teeth. Biochemical identification of pure cultures of streptococci, enterococci, and staphylococci was not performed. To identify the sensitivity of microflora to chlorhexidine, the crops on a Petri dish were treated with various concentrations of an antiseptic, and after 1 and 3 days the possible growth was analyzed. In both cases: when using 0.05% and 2% chlorhexidine, no growth of cultures was detected.
Analysis of the results obtained confirms the continued high activity of chlorhexidine against pathogens present in the carious cavity and their lack of resistance. The same final result obtained during the study using different concentrations of the antiseptic allows us to experimentally substantiate the feasibility of using chlorhexidine 0.05% in pediatric dentistry.
Possible side effects
As with any other drug, the use of chlorhexidine is fraught with side effects. People sensitive to the drug may experience allergic reactions.
Sometimes the effect of a solution or gel is manifested by photosensitivity - sensitivity of the skin and mucous membranes to ultraviolet radiation.
Rinsing with a solution is fraught with a change in the color of tooth enamel, the deposition of tartar, and the taste may also be affected.
sells antiseptics with chlorhexidine. We supply certified goods of our own production in bulk, guarantee quality and competitive prices, and organize delivery.