There are two types of regeneration - physiological and reparative. Physiological regeneration refers to the restoration of tissue structures of a healthy body as they age and die. A clear example of this is the skin - the constant peeling and exfoliation of the epidermis. Physiological regeneration is a constant and very slow process that does not cause a stressful situation in the body.
- Bone Regeneration: Basics
- Sources of regeneration
- Stages of reparative osteogenesis
- Means for stimulating osteoreparation
Bone Regeneration: Basics
Reparative regeneration is the restoration of damaged or lost tissue. The degree and quality of the regenerative process in different tissues is different. The higher the differentiation of the tissue (nervous, muscle), the less ability it has to restore its structure. Therefore, anatomical restoration of the damaged area occurs by replacing the defect with connective tissue - a scar. Damaged bone tissue is able to go through a number of stages of the reparative process and restore its anatomical shape, histological structure and functional suitability.
A bone fracture is accompanied by damage to adjacent soft tissues and causes a stressful situation, which is accompanied by local and general reactions of the body. During the process of bone tissue restoration, complex general and local biological and biochemical changes occur, which depend on the blood supply to the bone, the age of the patient, the general condition of the body, and the quality of treatment.
The effect of low frequency electrical stimulation on bone tissue regeneration
1.Clinic premorbid and emergency conditions FKU “MONKS” them. PV Mandryka MO, 121002 Moscow, Silver lane, 4. 2.FSBI research Institute of General pathology and pathophysiology, 125315 Moscow, the Baltic St.8. 3.FSBI Institute of biology of development. NK Koltsov Academy of Sciences, 119334, Moscow, Vavilov St, 26. 4.LLC "Medical center HuanDi"
The study was performed on 30 male rats of Wistar line (weight 330 — 360 g, age 3.5 months). In an experimental model of damage to the femur bone in the hip joint studied the effect of low frequency electrical stimulation of the damaged area on the rate of regeneration of bone. The animals were divided into two groups. Control (15 rats) and experienced (15 rats). In the experimental animals received stimulation of the injury site for 5 min daily for 7 days, 14 days and 21 days. Stimulation was carried out using a device “Osteon-1” generating a mixed signal of two voltage pulse of varying duty cycle, one of which is modulated to a higher frequency. Signals were not synchronized with respect to each other, unipolar with varying frequencies and amplitudes. The obtained results show the effectiveness of the electrical stimulation currents of low frequency in the restoration of bone tissue after damage. Morphological studies showed that electrical stimulation to accelerate the regeneration of damaged bone at all stages of the study (7,14,21 days), causes a more pronounced integration of newly formed bone with the old intact bone and promote the formation of more powerful periosteal calls in comparison with the control.
In most countries of the world, there is a significant decrease in mortality, as a result, an increase in life expectancy and, accordingly, an aging population [1]. However, improving health care does not mean improving the quality of life, both for an individual and for society as a whole. [2] . Unfortunately, the progress made in increasing life expectancy has not been matched by progress in reducing disability in older people.
It should be emphasized that the number of older people around the world is rapidly increasing. In particular, in the Russian Federation currently the share of old-age pensioners is 20.6% [3].
Osteoporosis is one of the most pressing problems of modern healthcare. In Russia, 14 million people over 50 years old suffer from osteoporosis. Another 20 million have osteopenia [4,5]. Osteoporosis ranks fourth in the frequency of disability after diseases of the cardiovascular system, diabetes and cancer.
According to WHO, about 75 million citizens of Europe, the USA and Japan suffer from this disease. Due to the aging population of Europe, by 2050 the number of osteoporotic fractures of the femoral neck is expected to increase from 500 thousand to 1 million cases annually [6. Osteoporosis is especially common in older women due to decreased levels of sex hormones. In addition to fractures, there is also a steady increase in degenerative diseases of the joints (osteoarthrosis, osteoporosis, aseptic necrosis). Joint diseases are accompanied by chronic pain. Studies conducted in the USA have shown that women experiencing pain fall 1.66 times more often [7], and this, in most cases with osteoporosis, inevitably leads to bone fractures.
The general pathogenetic mechanism of this group of diseases is a violation of the structure of bone tissue, accompanied by a complex local or systemic disruption of the processes of bone remodeling.
All of the above indicates that the problem of treating bone remodeling disorders is relevant and, due to the increasing aging of the population, is becoming increasingly relevant.
The accumulated experience in drug treatment of degenerative diseases shows that none of the currently existing drugs can reliably restore the quantity and quality of bone tissue [8]. In addition, one should take into account the fact that elderly and old people, as a rule, have a number of comorbid conditions and, accordingly, there is forced polypharmacy. Therefore, in recent decades, an intensive search has been carried out for the possibility of using not pharmacological, but physical methods to stimulate osteogenesis: alternating electromagnetic fields of high and low frequencies, direct electric current, ultrasound, which have a number of advantages in their practical application [9-11]. The advantages of these methods of influence are: safety, the possibility of long-term multifactorial effects, and lack of addiction.
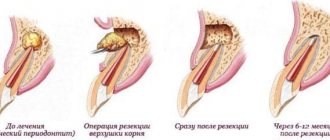
Experience in the treatment of necrosis of the femoral head has shown that external electrical signals can trigger a cellular response leading to reconstruction of the damaged bone [12-15].
Taking into account the results of numerous experimental and clinical studies indicating the effective effect of electrical stimulation on various body systems and, in particular, on reducing joint stiffness, muscle spasticity and suppressing pain after fractures, accelerating bone regeneration [9, 16-18], the Osteon device was developed -1" for effective restoration of bone tissue structure [13].
The purpose of this work is a histological study of the effectiveness of bone tissue restoration when stimulating damaged animal bones using the Osteon-1 device.
Sources of regeneration
Restoration of bone integrity occurs through the proliferation of cells of the osteogenic layer of the periosteum, endosteum, insufficiently differentiated pluripotent cells of the bone marrow, as well as due to metaplasia of giaraosseous tissues.
Modern ideas about the processes of bone tissue regeneration combine the concepts of neoplastic and metaplastic theories. Preosteogenic cells are considered osteoblasts, fibroblasts, osteocytes, pericytes, histiocytes, lymphoid, fat and endothelial cells, cells of the myeloid and erythrocyte series.
During the fusion of broken bones, a staged pattern of reparative osteogenesis has been established, which is conditional. The division into stages is not of fundamental importance, since they overlap in dynamics.
Even with ideal reposition and fixation of fragments, differentiation of various cells occurs simultaneously, and therefore the stages of the reparative process are difficult to distinguish. But to choose the optimal treatment tactics for patients, you need to have an idea of the patterns of reparative osteogenesis.
Methodology
The work was performed on 30 male Wistar rats (weight 330 - 360 g, age 3.5 months). The rats were kept under standard conditions, 5 animals per cage, with controlled temperature (24 oC) and lighting (for 12 hours) and with free access to water and food. The operation to simulate the injury was performed under general anesthesia. First, the animals were anesthetized with light ether anesthesia. For deeper anesthesia, chloral hydrate was used intraperitoneally at a dose of 300 mg/kg. Then the animal was fixed on the operating table, the hair in the left thigh area was trimmed, and the skin and muscle tissue was cut with a scalpel. The femur in the area of the hip joint was exposed, the femur was drilled to the medullary canal 8-10 mm distal to the hip joint using a miniature dental drill (bur diameter 0.8 mm). After bone damage, layer-by-layer suturing of soft tissues was performed. The animals were divided into two groups. Control (15 rats) and experimental (15 rats). In experimental animals, using the Osteon-1 device, stimulation of the injury area was performed for 5 minutes daily for 7 days. (op7), 14 days. (op14) and 21 days (op21). To do this, the animals were fixed on operating tables and a mixed signal of two pulse voltages of different duty cycles, one of which was modulated at a higher frequency, was supplied through surgical needles inserted under the skin. The signals were unsynchronized relative to each other, unipolar with varying frequencies and amplitudes. The cathode was located in the area of injury, the anode was placed distally on the same paw. Electrical stimulation was carried out under general anesthesia (chloral hydrate at a dose of 190-200 mg/kg, intraperitoneal).
Three groups of animals with trauma served as controls (k7, k14, k21); they were also placed on dissection tables; surgical needles were inserted into them, but stimulation was not carried out. In each experimental variant, 5 animals were used. The next day after the end of the study, the animals were removed from the experiment by cervical dislocation. Then the femurs of all 6 groups were removed, which were used for histological analysis and assessment of the severity of the pathological process and the process of osteogenesis.
Animal bones were fixed for 24 hours at room temperature in 10% formalin prepared in phosphate-buffered saline (PBS, 0.02 M, pH 7.6). They were decalcified in 5% trichloroacetic acid for 48 hours, then washed in PBS and frozen in isopentane at -400C. Next, sections with a thickness of 5 μm were prepared. The sections were dried at room temperature for 1 hour and stained with hematoxylin-eosin. For histological analysis, 100 to 200 serial sections of the femur were prepared from each animal in the transverse and longitudinal directions. Using an Olympus microscope (approx. 10x, ob. 4x). Microphotographs of bone tissue sections were taken. Morphometric analysis of bone tissue inside the wound channel (intermediary callus) was carried out using the ImageJ program [19]. The area of bone tissue measured on 3-5 sections from each animal was expressed as a % of the canal area. To determine the significance of differences between the experiment and the control, the nonparametric two-sided Mann-Whitney test (U-test) was used.
Stages of reparative osteogenesis
Stage of catabolism of tissue structures and cellular infiltration . Compared to inflammation, this is the stage of alteration (destruction). After injury, necrosis of damaged tissues and disintegration of the cellular elements of the hematoma occur.
The human body immediately responds to injury with a local phagocytic response. Along with this, decay products, which are genetic inducers, together with hormones determine the reproduction and proliferation of various specialized cells (osteocytes, histiocytes, fibrocytes, lymphoid, fat and endothelial cells), that is, small cell infiltration, which lasts 6-10 days.
cell differentiation stage lasts 10-15 days. Basically, DNA and RNA, as well as anabolic hormones, direct the differentiation of cells of the progressive small cell infiltrate. Three types of cell differentiation occur simultaneously: fibroblastic, chondroid and osteogenic. This depends on the conditions under which the reparative process occurs.
With ideal reposition and fixation of fragments and sufficient blood supply (use of hardware osteosynthesis, etc.), fusion occurs according to the type of primary osteogenesis. The differentiation of most cells is immediately aimed at the formation of osteoid tissue. When fixation is unreliable or there is insufficient blood supply to the fragments due to severe damage, cell differentiation occurs through fibrogenesis followed by metaply into cartilage and bone tissue.
The stage of primary osteon formation —the formation of an angiogenic bone structure—occurs within 16–21 days. It is characterized by the fact that complete revascularization of the primary callus occurs. The regenerate sprouts capillaries and mineralization of its protein base begins. A finely looped, chaotically oriented network of bone trabeculae appears, which gradually merge to form the primary osteon and Haversian tubules.
The stage of reconstruction of the primary regenerate or callus spongiosis is the stage at which lamellar bone tissue is formed. During the restructuring of the primary regenerate, the bone lamellar osteon gains orientation above the force lines of load, the cortical substance of the bone and periosteum appears and the bone marrow cavity is restored. The parts of the regenerate that are under load are resorbed. All this leads to complete restoration of the structure and function of the broken bone. Depending on the location of the fracture, the process of restructuring and recovery can last from several months to 2-3 years.
So, the following practical conclusions follow from the laws of reparative bone tissue regeneration:
1) ideal reposition and fixation of bone fragments should be achieved faster, and no later than the stage of cell differentiation begins;
2) late reposition, any intervention to correct fragments leads to repeated destruction of the capillaries of the regenerate and disruption of reparative osteogenesis;
3) the stimulator of lamellar bone formation in the process of restructuring the primary regenerate is the functional load, which should be remembered when treating patients.
Theoretically, there are three types of reparative regeneration of bone tissue - primary, primary delayed and secondary fusion. Primary bone fusion occurs within a short time by primary osteogenesis due to the formation of intermediary callus. But for this to happen, all conditions must be created. First of all, this is observed with downhole and compression fractures of bones, often after ideal reduction (diastasis between fragments of 50-100 μm) and reliable fixation of fragments.
Primary delayed fusion occurs when there are no gaps between fixed fragments, fusion occurs only through vascular canals (intracanalicular osteogenesis), i.e. partial fusion occurs, and complete interosseous fusion is preceded by resorption of the ends of the fragments. But from a practical point of view, this type of repair should be regarded as positive, and therefore clinicians adhere to the division into two types of bone restoration - primary and secondary.
Secondary fusion of fractured bones occurs due to the formation of less complete types of callus - periosteal, endosteal and paraosal (hematoma, soft tissue).
By forming excess periosteal and paraosal callus, the body tries to compensate for the fixation of fragments that the doctor did not do. This is the natural sanogenesis of the body. In this case, the period of bone fusion increases significantly. By the nature of the callus on the x-ray, you can immediately assess the quality of the patient’s treatment. The larger the callus, the worse the fixation of the fragments.
Secondary bone healing has been compared to the healing of soft tissue wounds. But there is a fundamental difference in the healing of damage to the two tissues. Healing of a soft tissue wound occurs by secondary intention and ends with the formation of a scar, while when a bone is fractured during the repair process, all bone cells go through the stage of metaplasia, which ends with the formation of full bone. However, in order for the bone to heal again, reliable fixation of the fragments is also necessary. If it is not there, then the cells will go through the stages of fibro- and chondrogenesis, the fracture will heal, but the bone will not heal.
The issue of stimulating reparative osteogenesis remains unresolved theoretically. There have been attempts to speed up the regeneration of bone tissue for a long time, and now the number of searches is not decreasing.
Features of the direction of bone regeneration (BRR)
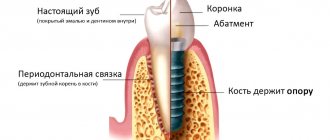
Guided bone regeneration is a surgical procedure that increases bone volume. During the operation, the hard part of the jaw is built up. As a result, its height and width increase.
All surgical techniques are in demand in prosthetics. By building up the hard part of the jaw, the doctor will be able to install a dental implant efficiently and reliably. A bone substitute is used to perform the procedure. With its help, the doctor will be able to give the bone the desired shape. The material is securely attached to the remnants of native bone tissue with a barrier membrane.
After this, the process of overgrowing with blood vessels and cells occurs, which are responsible for active bone growth. When the process is completed, the doctor will be able to fully install implants and dentures.
Examples of work “Before” and “After”
Restoration of all teeth on the upper and lower jaw - basal implantation
Case: partial absence of teeth on the upper and lower jaw, complicated by severe periodontitis (tooth mobility).
Complex one-stage implantation of the lower jaw
Case: there was a loose bridge of 4 front teeth on the lower jaw; after diagnosis, removal of the remaining teeth and coplex basal implantation were prescribed.
Restoration of all teeth using basal implantation method (March 2012)
Case: partial adentia, exposed roots of natural teeth, periodontitis, increased tooth mobility, severe atrophy of bone tissue in some places beyond the possible norms for classical dental implantation.
Restoration of anterior teeth using basal implantation method (April 2012)
Case: partial absence of front teeth and destruction of supporting teeth under the prosthesis, the relief of the gums and interdental papillae is disturbed.