In modern dentistry, a large number of drugs are used that help alleviate the course of a particular condition in the patient.
A special group includes drugs that are used for various forms of pulpitis to reduce pain. One of these drugs is Devit S.
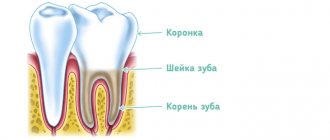
Devit S is a special paste that mummifies the pulp without using arsenic in the composition. The use of devitalizing paste promotes long-term effects on the blood vessels of the pulp without any pain or irritation.
Complete death of the neurovascular bundle occurs approximately 5-7 days after application. If the patient does not experience any discomfort, then the root canals can be filled with permanent materials within two days after using the paste.
Features of the composition
Devit S paste contains the following substances:
- paraformaldehyde, which is an antiseptic that destroys proteins and promotes devitalization of the pulp;
- lidocaine is a local anesthetic component that reduces pain that occurs during pulp mummification;
- creosote, which is an antimicrobial substance;
- paste former;
- fibrous component.
Release form: paste weighing 3 g.
Mechanism of action
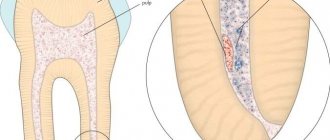
The death of the pulp under the influence of paraformaldehyde occurs due to impaired tissue respiration. Enzymatic systems are destroyed, as a result of which cells cannot function normally. As a result, the pulp becomes mummified and ceases to bother the patient.
The depth of influence of a substance is determined by the duration of its use. The longer the drug is applied, the more pulp tissue will die.
An anesthetic is used to reduce pain during the necrotizing effect of the paste. In addition, it helps relieve pain from the inflammatory process in the pulp that has already begun.
Instructions for use DEVIT 50 000 (DEVIT 50 000)
The drug is taken orally, regardless of food intake. The tablet should be swallowed whole (without chewing) and washed down with water.
For the treatment of vitamin D deficiency
prescribe 50,000 IU (1 tablet) per week for 7 weeks, followed by maintenance treatment (equivalent to 1400-2000 IU/day), for example, 1 tablet. per month, if necessary.
Maintenance therapy
is carried out under monitoring of the concentration of 25-(OH)-D in the blood over the next 3-4 months to confirm achievement of the target level.
The dose is adjusted individually depending on the severity of vitamin D deficiency and response to treatment.
For adjunctive therapy of osteoporosis
the drug is prescribed at a daily dose of 800-1000 IU or an equivalent total weekly dose of 7000 IU, or an alternative monthly dose of 50,000 IU vitamin D. For
elderly patients
, who are particularly at risk for falls and fractures, a dose equivalent to 2000 IU vitamin D per day is recommended . Patients should receive supplemental calcium if dietary intake is insufficient.
For vitamin D deficiency (serum levels of 25-(OH)-D <25 nmol/l or <10 ng/ml),
to adults and elderly patients
at the rate of 800-4000 IU/day for 12 weeks.
For vitamin D deficiency (serum 25-(OH)-D levels 25-50 nmol/l or 10-20 ng/ml)
,
long-term maintenance therapy after treatment of deficiency
in
adults and elderly patients
, as well as for the
prevention of vitamin D deficiency,
the drug is prescribed at a rate of 800-1600 IU/day.
For vitamin D deficiency or insufficiency
in
adolescents aged 12-18 years,
the drug is prescribed at the rate of 800 IU/day. Treatment is carried out only under the supervision of a doctor.
During vitamin D therapy, the level of calcium and phosphorus intake is of great importance for achieving the effect.
Before initiating vitamin D therapy, the patient's dietary habits should be carefully assessed and the addition of vitamin D-fortified foods should be recommended.
Certain populations are at high risk of vitamin D deficiency and may require higher doses and monitoring of serum 25-(OH)-D concentrations in these groups:
- persons suffering from alcoholism;
— institutionalized or hospitalized persons;
- dark-skinned;
— patients with diseases of the hepatobiliary system (impaired liver function, liver cirrhosis, obstructive jaundice);
- patients with malabsorption, incl. those suffering from inflammatory bowel disease, persistent diarrhea and celiac disease;
- obese patients;
— patients with diagnosed osteoporosis;
- patients using concomitant medications (for example, anticonvulsants, glucocorticoids);
- persons with limited exposure to the sun, incl. children;
- In general, vitamin D absorption will be impaired in any condition in which fat malabsorption occurs, such as steatorrhea.
Before starting treatment with vitamin D, it is necessary to assess the level of calcium and phosphate in the blood. To ensure the effectiveness of treatment, it is necessary to ensure adequate calcium intake from food.
Due to variation in individual sensitivity to vitamin D, the dose may be adjusted based on clinical effectiveness.
When choosing a dose of vitamin D, it is necessary to take into account the amount of vitamin D intake from other sources.
In patients undergoing hemodialysis
, to control elevated phosphate levels in plasma, it is possible to use phosphate binding agents (phosphate binders). During vitamin D therapy, it may be necessary to increase the dose of phosphate binders due to improved phosphate absorption. The product of calcium and phosphorus concentration (Ca×P, mg/dL) should not exceed 60.
Vitamin D deficiency due to malabsorption or liver disease often requires higher doses for treatment, up to 1 mg (40,000 IU) daily. Doses up to 2.5 mg (100,000 IU) may be used to treat hypocalcemia due to hypoparathyroidism.
Patient monitoring
The following indicators may be useful in monitoring patients (other tests may be required depending on the patient's condition):
- blood calcium concentration or plasma ionized calcium level. Due to the narrow therapeutic range, it is recommended that these parameters be determined at least once a week during the early period of treatment and then periodically during treatment. If possible, it is worth determining the concentration of both free and bound calcium in the blood;
- the level of alkaline (alkaline) phosphatase activity in the blood plasma, the concentration of phosphates in the blood, the concentration of calcium in 24-hour urine, the ratio of calcium and creatinine in the urine. It is recommended to determine these indicators every 1-3 months during therapy;
- plasma urea nitrogen and plasma creatinine. Periodic monitoring is recommended in patients receiving the drug in therapeutic doses.
Special patient groups
Devit 50,000 is not recommended for children under 12 years of age
due to the lack of ability of many to swallow a tablet safely.
Research shows that in older patients
the need for vitamin D may be increased due to a decrease in the ability of the skin to synthesize previtamin D3 or decreased sun exposure, or weakened renal function, or decreased absorption of vitamin D. To select a dose, it is necessary to focus on the concentration of 25-hydroxycholecalciferol in the blood plasma.
Vitamin D deficiency in the body due to liver diseases
often requires higher doses for treatment. To select the dose, it is necessary to focus on the concentration of 25-hydroxycholecalciferol in the blood plasma.
Features of application
The method of using Devit S paste is as follows:
- Conducting anesthesia.
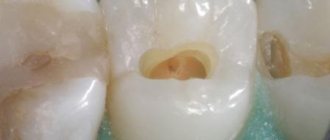
- Opening the carious cavity and completely removing necrotic dentin.
- Point opening of the pulp chamber.
- Using a thin trowel or probe, a devitalizing paste is applied to the exposed pulp horn. The approximate volume should be equal to a grain of rice. It is recommended to apply 2 times less of the drug to baby teeth than to permanent teeth.
- Then a small cotton ball is placed on the paste, which will help cushion the filling material at the site of application and thereby reduce pain.
- Finally, the carious cavity is hermetically sealed with filling material.
The first examination of the patient is scheduled after 2 days. If there is no pain, further root canal treatment is performed. If the unpleasant sensations persist, then you need to walk with the paste for another 2-3 days until the pulp completely dies.
When using pastes that do not contain arsenic, there is no need to additionally rinse the root canals with special neutralizing substances.
Devit-S (potent paste) (3g)
DEVIT - S - POWERFUL DEVITALIZING PASTE WITHOUT ARSENIC
PURPOSE
- Paste "Devit-S" is used for pulp devitalization in the treatment of pulpitis using the method of mortal extirpation or amputation, for the treatment of residual root pulpitis of temporary and permanent teeth, and also as an additional means for devitalization during a repeated procedure after the use of pastes containing arsenic.
BASIC PROPERTIES
Arsenic-free paste "Devit-S" contains:
- paraformaldehyde is an antiseptic that coagulates albumin, providing pulp devitalization;
- lidocaine hydrochloride, which is a local anesthetic and reduces the risk of painful reactions;
- creosote is an antimicrobial component;
- paste former and filler, giving the paste a fibrous structure.
The use of Devit-S paste ensures prolonged devitalization of the pulp with virtually no irritation and pain, and also eliminates the need for additional treatment of the canals to neutralize arsenic salts, as is the case with the use of arsenic pastes.
MODE OF APPLICATION
- Clean the cavity from carious dentin and open the pulp chamber.
- Introduce the required amount of paste using a probe (without pressure) into a well-opened (or open) cavity. In case of significant necrosis of the pulp surface, it is necessary to remove part of the necrotic pulp under application anesthesia.
- The amount of paste is determined individually.
- When treating pulpitis of baby teeth, as well as single-rooted teeth in adults, it is enough to apply the paste in an amount equal to the size of millet grain.
- To devitalize the pulp of multi-rooted teeth, the amount of paste must be doubled.
- After applying the Devit-S paste, it is necessary to hermetically close the cavity with a temporary filling material (water-based Dentin powder).
- Complete devitalization of the pulp occurs in 3-5 days, in rare cases - in 7 days.
- If the patient does not feel pain, permanent filling can be performed within 24-48 hours after applying the paste.
- If direct contact with the pulp is not achieved, devitalization must be carried out in two stages. Direct contact can only be achieved in the second stage after pulp viability has decreased.
- After devitalization and extirpation of the pulp, instrumental and medicinal treatment of the canal is necessary (a set of EndoZhi fluids).
Precautions: The patient should be instructed to immediately seek medical attention if they experience a creosote taste in the mouth, which indicates leakage of the devitalizing paste through the temporary filling.
Note: The drug is contraindicated for persons with hypersensitivity to paraformaldehyde. If pulpal pain intensifies after applying the paste (the pulp horn is not exposed or the paste is applied very tightly), it is necessary to perform infiltration anesthesia with lidocaine.
RELEASE FORM
- Paste (syringe) - 3.0 g
STORAGE CONDITIONS Store in a dry place, protected from direct sunlight, in a tightly closed container, at a temperature from +5°C to +20°C. Seal tightly after each use.
Shelf life – 2 years.
Precautionary measures
The patient must be warned that if any uncharacteristic taste appears in the oral cavity, it is necessary to immediately consult a doctor, as there is a possibility of devitalizing paste leaking through the filling.
If the permissible period of action of the drug is exceeded, local toxic effects may develop. The gums around the tooth change its color, and the pain changes its character. Severe discomfort appears when biting, which indicates the progression of toxic periodontitis.
Adverse reactions
If the drug is used carelessly and incorrectly, the following complications may develop:
- destruction of bone tissue;
- damage to the jaw nerves;
- burns of the oral cavity;
- necrotic changes in the gingival papillae;
- constant pain, which only intensified after using the paste;
- When treating baby teeth, there is a risk of damage to the permanent tooth germ.
If such phenomena occur, it is necessary to immediately remove the temporary filling and remove the drug. The root canals are washed with antidite and antiseptics. The mucous membrane is treated with healing substances.
Arsenic-free pastes
With pastes without arsenic, the situation is much simpler: even with prolonged use, periodontitis does not occur so quickly, although you should not exceed the time prescribed by the doctor!
Arsenic-free pastes are even used to treat children. Thus, the treatment is intermittent and the child does not have to stay in the chair for a long time. And also, which is very important, it is possible to do without injections.
For the adult population, root canal treatment is successfully carried out under anesthesia, on average in 1-3 visits, depending on the complexity of the situation.
Cost and analogues
The price of Devit S ranges from 100 to 130 rubles per syringe; analogues are also available for purchase:
- Depulpin . Produced in Germany.
- Caustinerf forte . Produced in France.
- Non-arsenic . Produced in Russia.
- Devitec AS . In production.
- Devital . Produced in Russia.
- Devit ARS . Arsenic-based analogue. The disadvantages of its use are such as high toxicity and the impossibility of using it in the treatment of baby teeth. Also produced.
- Devit P. Used in children. Instead of creosote, the composition contains camphor, menthol and chlorophenol.
All analogues presented have a higher cost, although their effectiveness is the same as that of Devit S.
Devit 50000 tablets p/o 50000ME No. 8x1
Name
Devit 50000.
Description
Reddish-brown oval film-coated tablets.
Main active ingredient
Vitamin D
Release form
Pills.
Dosage
50 thousand IU.
pharmachologic effect
Vitamin-calcium-phosphorus metabolism regulator.
Pharmacological group
Vitamins. Vitamin D and analogues.
Pharmacological properties
Pharmacodynamics
Vitamin D3 regulates the exchange of calcium and phosphorus in the body, accelerates the absorption of calcium in the intestines, improves the reabsorption of calcium and phosphorus in the kidneys, maintains the necessary level of these elements in the blood, promotes the formation of the bone skeleton, as well as the preservation of bone structure. The concentration of calcium ions in the blood determines the maintenance of muscle tone of skeletal muscles, myocardial function, promotes nervous stimulation, and regulates the process of blood clotting. Vitamin D3 is also necessary for the normal functioning of the parathyroid glands. With aging, the time spent in the sun and the skin's ability to synthesize vitamin D3 decreases. Due to weakening kidney function, the level of the active metabolite of vitamin D produced in the kidneys, 1,25(OH)2D, decreases, which contributes to the widespread prevalence of vitamin D deficiency among older people. Since vitamin D is necessary for sufficient calcium absorption and normal bone metabolism, its chronic deficiency causes secondary hyperparathyroidism and, as a consequence, activation of bone metabolism and rapid loss of bone mass. Taking vitamin D, especially when combined with calcium, slows bone loss and reduces the incidence of fractures. Vitamin D therapy is a mandatory component of the prevention of osteoporosis, along with calcium intake. In addition, taking vitamin D is a mandatory component of complex treatment of established osteoporosis.
Pharmacokinetics
The pharmacokinetics of vitamin D have been well studied. Vitamin D is well absorbed from the gastrointestinal tract in the presence of bile. It is hydroxylated in the liver to form 25-hydroxycholecalciferol and then undergoes further hydroxylation in the kidney to form the active metabolite 1,25-hydroxycholecalciferol (calcitriol). These metabolites circulate in the blood, binding to specific alpha globulin. Vitamin D and its metabolites are excreted from the body, mainly with bile and feces. Features of the pharmacokinetics of the drug in certain groups of patients In patients with impaired renal function, a 57% decrease in the rate of metabolic clearance was observed compared with the same indicator in healthy volunteers. Volunteers with malabsorption syndrome experienced decreased absorption and accelerated excretion of vitamin D. Volunteers who are obese are less able to maintain vitamin D levels in the body when exposed to sunlight, requiring higher doses.
Indications for use
Treatment of vitamin D deficiency. Prevention of vitamin D deficiency in high-risk patients.
Directions for use and doses
Treatment of vitamin D deficiency: 50,000 IU/week (1 tablet) for 7 weeks, followed by maintenance treatment (equivalent to 1400–2000 IU/day), for example, 1 tablet per month, if necessary. Maintenance therapy: is carried out under the control of the concentration of 25-(OH) D in the blood over the next 3-4 months to confirm the achievement of the target level. The dose is individualized depending on the severity of vitamin D deficiency and response to treatment, for example: Osteoporosis: for adjunctive therapy of osteoporosis at a daily dose of 800–1000 IU, or an appropriate total weekly dose of 7000 IU, or an alternative monthly dose of 50,000 IU vitamin D. For For elderly patients who are particularly at risk of falls and fractures, a dose equivalent to 2000 IU of vitamin D per day is recommended. Patients should receive supplemental calcium if dietary intake is insufficient. Vitamin D deficiency (serum 25-(OH)D levels
Use during pregnancy and lactation
There are limited data on the use of cholecalciferol in pregnant women. Animal studies have shown reproductive toxicity. During pregnancy, taking vitamin D in high doses is not recommended due to the possibility of a teratogenic effect in case of overdose. Also, overdose of vitamin D should be avoided in pregnant women, as long-term hypercalcemia has sometimes been associated with slow physical and mental development, supravalvular aortic stenosis, and retinopathy in the child. During breastfeeding, it is necessary to take the drug with caution, since when the mother takes a large dose of the drug, symptoms of hypercalcemia in the child are possible.
Precautionary measures
Vitamin D should be used with caution in patients with impaired renal function, and monitoring of calcium and phosphate levels is recommended while taking the drug. The risk of soft tissue calcification should be considered. Since vitamin D in the form of cholecalciferol cannot be metabolized normally in patients with severe renal failure, other forms of vitamin D should be prescribed to such patients (see section "Contraindications"). Caution should be exercised when using vitamin D in patients with cardiovascular diseases receiving treatment with appropriate drugs (cardiac glycosides, diuretics). Cholecalciferol should be administered with caution to patients with sarcoidosis due to the risk of increased metabolism of vitamin D to its active form. In such patients, monitoring of calcium levels in serum and urine is necessary. Concomitant use of multivitamin preparations and dietary supplements containing vitamin D should be avoided. High-dose oral vitamin D (500,000 IU single bolus) has been reported to increase the risk of fractures in older adults, with the greatest increase occurring within the first 3 years of age. months after taking the drug. Vitamin D treatment may identify previously undiagnosed primary hyperparathyroidism. Corrected serum calcium levels should be checked 1 month after completion of the exercise regimen or after initiation of vitamin D supplementation if primary hyperparathyroidism is detected. During treatment, it is necessary to regularly monitor plasma calcium concentrations (initially weekly, then once every 2–4 weeks). During long-term treatment, in addition, urinary calcium excretion and renal function should be monitored by measuring serum creatinine levels. Monitoring is especially important in elderly patients who are concomitantly taking cardiac glycosides or diuretics and in cases of hyperphosphatemia, as well as in patients at increased risk of stone formation. In case of hypercalciuria (more than 300 mg (7.5 mmol)/24 hours), or if there are signs of renal impairment, the dose should be reduced or treatment discontinued. Similar monitoring is necessary for children whose mothers were treated with vitamin D in pharmacological doses. Some children may react with increased sensitivity to the effects of vitamin D. The tablets should not be taken in cases of pseudohypoparathyroidism (vitamin D requirements may sometimes be reduced by normal sensitivity to vitamin D, with a risk of overdose with long-term use). In such cases, other vitamin D derivatives are available. Interactions with other drugs Concomitant use of anticonvulsants (eg, phenytoin), hydantoin, primidone, or barbiturates (and possibly other drugs that cause hepatic enzyme induction) may reduce the effect of vitamin D by metabolic inactivation. Concomitant use of glucocorticoids may reduce the effect of vitamin D. Systemic corticosteroids inhibit calcium absorption. Long-term use of corticosteroids may be offset by the effects of vitamin D. In cases of treatment with drugs containing digitalis or other cardiac glycosides, concomitant use of vitamin D may increase the risk of cardiac glycoside toxicity (arrhythmias). Strict medical supervision is required, as well as monitoring of serum calcium concentrations and ECG, if necessary. Concomitant use of ion exchange resins such as cholestyramine or laxatives such as paraffin oil may reduce the gastrointestinal absorption of vitamin D. Thiazide diuretics reduce urinary calcium excretion. Taking large amounts of vitamin D along with diuretics can lead to excess calcium in the body. In cases of simultaneous treatment with thiazide diuretics, which reduce urinary calcium excretion, monitoring of serum calcium concentration is recommended. The cytotoxic agent actinomycin and imidazole antifungals interact with vitamin D to inhibit the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D by the kidney enzyme, 25-hydroxyvitamin D-1-hydroxylase. Use of calcium-containing products in large doses may increase the risk of hypercalcemia. Avoid taking products containing magnesium (such as antacids) as this may lead to a risk of hypermagnesemia. Vitamin D increases the absorption of drugs containing phosphorus and the risk of hyperphosphatemia. Concomitant use of calcitonin, etidronate, gallium nitrate, pamidronate, plicamycin with vitamin D may antagonize the effect of these drugs in the treatment of hypercalcemia.
Interaction with other drugs
Concomitant use of anticonvulsants (eg, phenytoin), hydantoin, primidone, or barbiturates (and possibly other drugs that cause hepatic enzyme induction) may reduce the effect of vitamin D through metabolic inactivation. Concomitant use of glucocorticoids may reduce the effect of vitamin D. Systemic corticosteroids inhibit calcium absorption. Long-term use of corticosteroids may be offset by the effects of vitamin D. In cases of treatment with drugs containing digitalis or other cardiac glycosides, concomitant use of vitamin D may increase the risk of cardiac glycoside toxicity (arrhythmias). Strict medical supervision is required, as well as monitoring of serum calcium concentrations and ECG, if necessary. Concomitant use of ion exchange resins such as cholestyramine or laxatives such as paraffin oil may reduce the gastrointestinal absorption of vitamin D. Thiazide diuretics reduce urinary calcium excretion. Taking large amounts of vitamin D along with diuretics can lead to excess calcium in the body. In cases of simultaneous treatment with thiazide diuretics, which reduce urinary calcium excretion, monitoring of serum calcium concentration is recommended. The cytotoxic agent actinomycin and imidazole antifungals interact with vitamin D to inhibit the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D by the kidney enzyme, 25-hydroxyvitamin D-1-hydroxylase. Use of calcium-containing products in large doses may increase the risk of hypercalcemia. Avoid taking products containing magnesium (such as antacids) as this may lead to a risk of hypermagnesemia. Vitamin D increases the absorption of drugs containing phosphorus and the risk of hyperphosphatemia. Concomitant use of calcitonin, etidronate, gallium nitrate, pamidronate, plicamycin with vitamin D may antagonize the effect of these drugs in the treatment of hypercalcemia.
Contraindications
Hypersensitivity to vitamin D or components of the drug. Hypervitaminosis D. Renal osteodystrophy with hyperphosphatemia. Hypercalcemia and/or hypercalciuria. Urolithiasis, the presence or risk of calcium kidney stones. Severe renal failure. Children's age up to 12 years.
Compound
Each film-coated tablet contains: Active ingredients: 50,000 IU vitamin D3 (equivalent to 1.25 mg cholecalciferol) 500 mg; Auxiliary ingredients: microcrystalline cellulose PH 102:290, hydrated colloidal silicon dioxide, anhydrous dicalcium phosphate, magnesium stearate, croscarmellose sodium; Shell: hydroxypropyl methylcellulose, microcrystalline cellulose, stearic acid, titanium dioxide E171, red iron oxide E172.
Overdose
Excessive vitamin D intake leads to hypercalcemia and its associated effects, including hypercalciuria, ectopic calcification, renal damage, and cardiovascular damage. Symptoms of overdose include: anorexia, fatigue, nausea and vomiting, diarrhea, polyuria, sweating, headache, thirst and dizziness, bone pain, arrhythmias, coma and even death (with severe hypercalcemia), irreversible kidney damage (with persistent hypercalcemia). Individual vitamin D intolerance varies widely; neonates and children tend to be more sensitive to its toxic effects. Recommended Treatment: Vitamin D hypervitaminosis is treated with vitamin withdrawal, a low-calcium diet, and plenty of fluid intake. If hypercalcemia persists, you can switch to a low-calcium diet and start prednisolone. Severe hypercalcemia can be treated with calcitonin, etidronate, pamidronate, or gallium nitrate. A hypercalcemic crisis requires intensive hydration with normal saline to increase calcium excretion, with or without the use of loop diuretics. Cardiac arrhythmias can be treated with low doses of potassium with continuous cardiac monitoring. Carcinogenicity, mutagenicity, reproductive dysfunction It has been established that cholecalciferol in high doses (4–15 times higher than the permissible dose for humans) has a teratogenic effect in animals. The offspring of pregnant female rabbits given high doses of vitamin D had anatomical lesions identical to those of supravalvular aortic stenosis, and the offspring that did not show such changes had vasculotoxicity similar to that seen in adults. for acute vitamin D intoxication.
Side effect
Like all medicines, Devit 50,000 may cause side effects. Side effects associated with the use of Devit 50,000 may include: Uncommon side effects (less than 1 in 100 people) Excess calcium in the blood (hypercalcemia). Feeling nauseous, loss of appetite, constipation, abdominal pain, extreme thirst, muscle weakness, drowsiness, or confusion. Excess calcium in urine (hypercalciuria) Rare side effects (less than 1 in 1000 people) Skin rash, itching, urticarial rash, facial swelling, genital swelling, dry skin, nail lesions, dry mouth, loss of appetite, erythematous rash, rash purpura , shortness of breath and dysphagia. Growth retardation may occur in children, especially after taking cholecalciferol for an extended period of time at a dose of 45 mcg (1800 units) per day. Early symptoms of vitamin D hypervitaminosis caused by hypercalcemia: constipation, usually more common in children and adolescents; diarrhea; dry mouth; prolonged headache; increasing thirst; increased frequency of urination, especially at night, or increased volume of urine produced; loss of appetite; metallic taste in the mouth; nausea or vomiting (more common in children and adolescents); unusual tiredness or general weakness. Late symptoms of vitamin D hypervitaminosis caused by hypercalcemia: bone pain; cloudy urine; arterial hypertension; photosensitivity or eye irritation; arrhythmia; itchy skin; lethargy (drowsiness); muscle pain; nausea or vomiting, pancreatitis (severe abdominal pain); psychosis, mood swings or mental changes; fast weight loss. If the listed adverse reactions occur, as well as reactions not listed in the instructions, the patient is advised to contact his doctor.
Storage conditions
Store in a place protected from moisture at a temperature not exceeding +30 °C. Keep out of the reach of children.
Buy Devit 50000 tab. p/capt.ob. 50,000 IU per bl. in pack No. 8x1 in the pharmacy
Price for Devit 50,000 tab. p/capt.ob. 50,000 IU per bl. in pack №8x1
Instructions for use for Devit 50000 tablets. p/capt.ob. 50,000 IU per bl. in pack №8x1